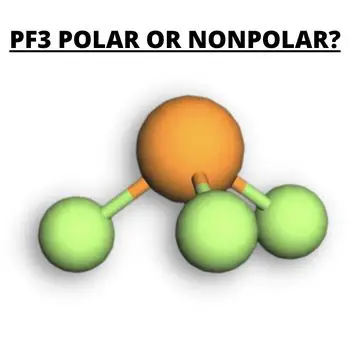
PF3 is an odorless and colorless chemical compound. It has slow hydrolysis compared to other chemical compounds.
Some may still be confused if PF3 is polar or nonpolar. So today, we will cover a topic on the polarity of this molecule.
Table of Contents
Polarity or Nonpolarity of Phosphorus Trifluoride
Molecules can either be polar or nonpolar, depending on the characteristics of the chemical compound. The Phosphorus trifluoride has Phosphorus as the central atom with three Fluorine atoms. The three atoms of Fluorine are bonded with Phosphorus leaving one lone pair of electrons completing the octet rule of the polar.
The geometrical shape of PF3 is trigonal pyramidal with an angle of 96.3 degrees. The polar molecule will result in an asymmetric shape.
The polar molecules like PF3 are affected due to the polar bonds, geometrical shape, electronegativity, and dipole moment of the molecule.
What Are Its Properties
The chemical formula for Phosphorus Trifluoride is PF3. The polar compound is a clear and odor-free gas that reacts slowly with water. The molecular weight of PF3 is 89.96 g/mol and has a density of 3.91 g/L. The melting point of PF3 is −151.5 °C, and the boiling point is −101.8 °C. In addition to this, PF3 is moisture and oxygen-sensitive.
This polar compound is a compressed, corrosive, and acutely toxic gas [1]. The chemical compound contains gas under pressure that may explode when heated. Since it is acutely toxic, it is fatal when inhaled and may cause severe skin burns and eye damage when exposed.
Uses of PF3
The most common use of PF3 is ligand in metal complexes. It is a molecule that binds the central atom P to form a coordination complex. With metal bonding, the PF3 is involved in the formal donation of two or more electron pairs with Lewis Bases.
PF3 is used as a ligand with metal carbonyls wherein there is a transition of metals with carbon monoxide. Carbonyls are helpful in organic syntheses, such as hydroformylation.
How to Determine PF3 Polarity

Geometrical Shape
To identify if the compound is polar molecules, you can check the molecular geometry that it produces. The Fluorine has seven valence electrons in the outermost shell, while the Phosphorus has 5. The geometrical shape of Phosphorus trifluoride is trigonal pyramidal, so PF3 is a polar particle.
Based on VSEPR Theory, polar molecules like PF3 are polar due to the lone pair that creates repulsion. The two atoms bond pairs that are polar will produce an asymmetrical bond angle at approximately 96.3 degrees. Nonpolar molecules have symmetric shapes, and the structure of PF3 is asymmetric, making PF3 a polar molecule.
Electronegativity
For the electronegativity of the PF3, Fluorine has a more electronegative atom compared to Phosphorus. The two poles will gain partial negative charge and partial positive charge that nonpolar compound does not have. The more electronegative atom attracts the charge, which makes a molecule polar.
The Fluorine atoms form 3.98 electronegativity, and Phosphorus has 2.19. To find the difference of electronegativity between Fluorine and Phosphorus, subtract the electronegativity. If the difference is higher than 0.5, the molecules are polar. Due to the difference of electronegativity of 1.88 between the molecules, the bonded atoms are polar.
Dipole Moment
Another indicator if the compound is a polar or nonpolar molecule is by the dipole moment. The moment is directly proportional to the molecule’s polarity, so nonpolar molecules have zero dipole moment.
The mathematical equation to calculate the moment for PF3 is D= Q+ R. Q is the magnitude of the charge, and R is the distance between the center of the negative and positive charges. The dipole moment of PF3 is 1.03D, making it a polar molecule.
FAQS
Yes, PF3 has a dipole moment. PF3 does not contain hydrogen atoms, and with this, it does not have any possibility of forming oxygen and hydrogen bonds. Since PF3 has lone pairs, there will be a non-zero dipole moment which makes the compound polar. In addition to this, there will be dispersion forces between pairs of molecules resulting in a permanent dipole of polar particles
PF3 is a covalent bond. After it forms three single bonds, the P will have one lone pair that will force the attraction between the two bonded pairs.
A molecule’s strongest bond can be by covalent or ionic bonds. The best example of ionic is sodium chloride that transfers one electron to another atom. Since there will be no transfer of electrons, Phosphorus Trifluoride has covalent bonds like Phosphorus Trichloride and Silicon Tetrafluoride.
So, Is PF3 Polar or NonPolar?
PF3 is a polar molecule. The chemical compound contains one lone pair of electrons in Phosphorus and three Fluorine atoms that create polar bonds. Phosphorus trifluoride has a trigonal pyramidal shape because of its electronegativity difference. The moment is non zero and the polar bond dipoles, so PF3 is a polar molecule.
With these characteristics, the entire molecule is polar.
Thank you for reading this far! I hope that the information provided in this article will be helpful to you.
References:
- https://pubchem.ncbi.nlm.nih.gov/compound/Phosphorus-trifluoride#section=Structures