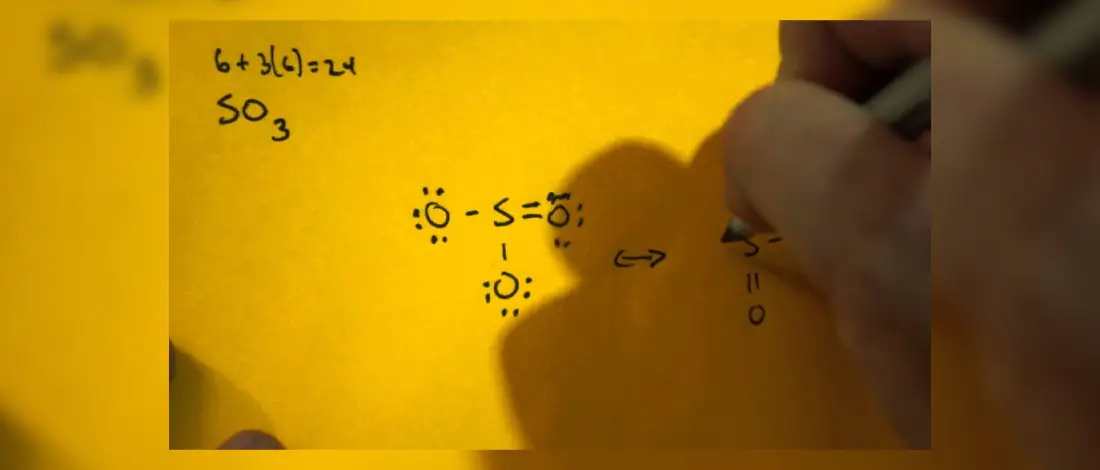Ethyne (C2H2), commonly known as Acetylene, is a gaseous alkyne hydrocarbon. It is a chemical compound widely used as a fuel in oxyacetylene welding and cutting metals.
Knowing the C2H2 Lewis Structure allows us to understand this chemical compound’s chemical properties and the shape of the molecules.
Lewis Structure of C2H2 Explained
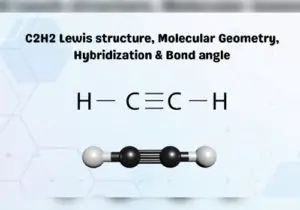
C2H2 consists of a simple structure of two Carbon atoms and two hydrogen atoms.
All four atoms are in a single line with one Hydrogen atom bonded at each end, while the Carbon atoms are in the center with a triple bond.
The Hydrogen and Carbon atoms are positioned linearly with a symmetrical arrangement on both sides.
It is classified as linearly aligned with 180° along its axis.
This molecule is also nonpolar due to having an electronegativity difference of 0.35 and a net-zero dipole moment across the molecule.

Understanding Its Properties and Structure
Lewis Structure of C2H2
It’s important to know the electronic configuration of Carbon (C) and Hydrogen to determine the Lewis Structure of C2H2. Carbon (C) has an electronic configuration of 1s2 2s2 2p2. Its atomic number is 6. Meanwhile, Hydrogen (H) has an electronic configuration of 1s1.
As per the octet rule, an atom can stabilize by stabilizing the number of valence electrons. There are eight valence electrons for Carbon and two valence electrons for Hydrogen. T
Shape of C2H2
The structure of the C2H2 has a basic linear shape. The Carbon atoms and Hydrogen atoms are bonded and arranged in a straight line. The atom arrangement is symmetrical in the center and on any side of the molecule.
Valence Electrons of C2H2
The Hydrogen atoms only need one valence electron to attain a stable lewis structure. Bonding with the Carbon atom, the Hydrogen atoms will share one valence electron of each Carbon atom.
The octets of both the Hydrogen atoms are complete leaving only the Carbon atoms with an incomplete octet. The Carbon atoms form a triple bond as they share their remaining three valence electrons to attain a stable condition.
Hybridization of C2H2
Sp is the hybridization of carbon atoms in Acetylene (C2H2).
Moreover, there are unhybridized 1s atomic orbitals in the hydrogen atoms that have unhybridized 1s atomic orbitals.
In sp hybridization, the s orbital of the central atom can only bind with one of its p orbitals.
There will always be a linear molecular geometry in atoms that show sp hybridization.
Therefore, two sp orbitals will be held at 180° to each other.
Molecular Geometry of C2H2
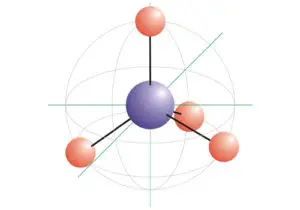
Acetylene (C2H2) is a tetra atomic molecule with two different atoms that bond in equal numbers. Additionally, Acetylene (C2H2) has a linear structure and a bond angle of 180° because of the Carbon bonding to the other Carbon atom.
Valence Shell Electron Pair Repulsion theory states that the valence electrons surrounding an atom in the pair tend to repel each other until they reach an arrangement where this repulsion is minimized.
Also Read:
Bond Angle of C2H2
The Carbon and Hydrogen atoms are positioned on the same plane.
Both the Carbon atoms serve as the center atom and are bonded with each other.
The Hydrogen atom is bonded to each of the ends of the Carbon atom, forming a linear bond formation with an angle of 180°.
Polarity of C2H2
C2H2 is nonpolar because the electronegativity difference between Carbon and Hydrogen is 0.35. The electronegative difference between two constituent elements should be greater than the value of 0.4.
The nature of C-H bonds is nonpolar covalent. This makes the complete C2H2 molecule a nonpolar molecule. It also has a net-zero dipole moment across the molecule.
Molecular Orbital (MO) Diagram of C2H2
The basic principle of The Molecular Orbital (MO) Diagram is that atomic orbitals combine and overlap to form a similar number of molecular orbitals. This happens when electrons move to different orbitals as electrons get excited. A molecular diagram specific to each molecule in the periodic table is born when electrons move from their original positions and form bonding and antibonding orbitals.
Drawing the C2H2 Lewis Structure
Distribute the electrons around the central atom to fill the outer shell of each atom. The C2H2 has a total number of 10 valence electrons. Place the least electronegative atom in the center and the Hydrogen outside.
Place the two electrons between atoms to form a bond and complete octets on the outside atoms. If the central atom does not have an octet, move electrons from outer atoms to form double or triple bonds.
FAQS
C2H2 is linear. It consists of both the Carbon atoms taking the central position. The two hydrogen atoms form single bonds at the ends of each Carbon atom, forming a line.
C2h2 is triple bonded because Carbon atoms share their remaining three valence electrons to attain a stable condition.
Key Takeaways
Acetylene [1] is the simplest member of the hydrocarbon series, which contains one or more pairs of carbon atoms that are linked by triple bonds. Understanding the properties, such as molecular geometry, hybridization, polarity, and molecular orbital, can help understand the behavioral properties of the C2H2 molecule.
In conclusion to this article, C2H2 is a linear molecule consisting of two carbon atoms and two hydrogen atoms. It has an sp hybridization and a bond angle of 180°. Ethyne has a ten total number of valence electrons. Also, there are no unshared electrons because the valence electrons are used up in the structure.
Thank you for reading this far! I hope that the information provided in this article will be helpful to you.
Reference:
- https://www.britannica.com/science/acetyleneIntermolecular-Force.pdf