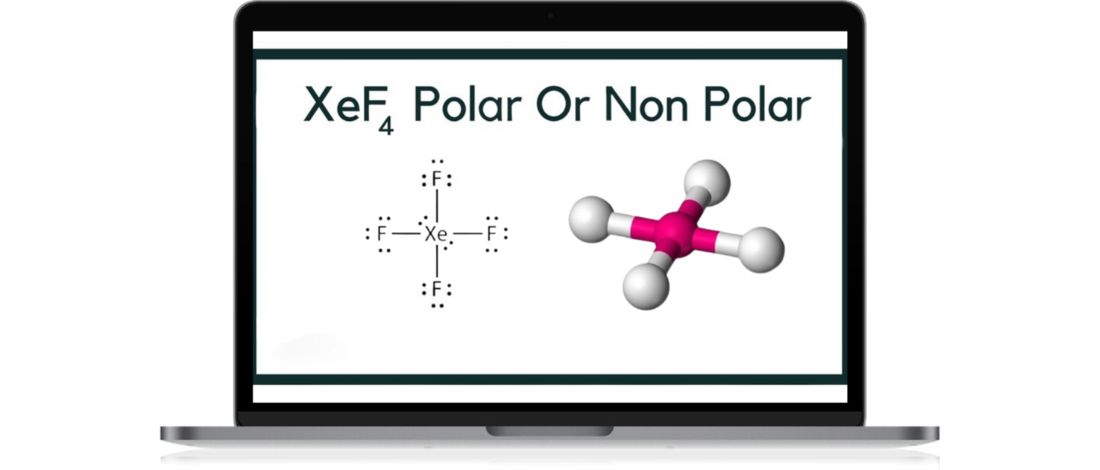
The polarity of Xenon Tetrafluoride created debate with every Science enthusiast. The reason behind this is that the polarity of XeF4 can be quite confusing, as some of its properties can be polar or nonpolar.
So, is XeF4 polar or nonpolar?
Let’s discuss the factors to correctly determine the polarity of XeF4.
Table of Contents
Determining the Polarity or Nonpolarity of XeF4
XeF4 is a chemical compound composed of a Xenon atom and Fluorine atoms. XeF4 is widely used to degrade silicone rubber because XeF4 leaves a residue of metal impurities. There are twelve atoms in the central Xe atom, wherein the eight atoms share the four Fluorine bonds. The two lone pairs of electrons, on the other hand, are nonbonding electrons.
The central atom Xenon is bonded chemically with four fluorine atoms creating a square planar molecular geometry [1].
Although the individual Xe-F are polar, the Xenon Tetrafluoride is a nonpolar molecule because it cancels each other, making the net dipole moment zero.
There are four Xe-F bonds, and each has individual bond dipoles with direction and magnitude. Since it creates molecules in a symmetrical shape, XeF4 is nonpolar.
Molecular Structure & Formation
The chemical compound Xenon Tetrafluoride forms when a Xenon as the central atom reacts with Fluorine atoms. When the four electrons bond with the half-filled orbitals, it is placed on either side of the atom. There will be octahedral geometry of the molecule when the hybridization of Xenon and Fluorine takes place. The Xe valence shell consists of six electrons in 5p orbital and two electrons in 5s. When the two electrons in the excited state fill the vacancies, there will be sp2d2 hybridization.
On the other hand, the formation process of Xenon Tetrafluoride is exothermic and releases 251 kJ/mol net energy.
How to Determine Polarity
Shape
Xenon Tetrafluoride combines noble gas Xenon and Fluorine with the chemical equation Xe + 2 F2. To define the physical structure of XeF4, draw the Lewis Structure of the XeF4. There are a total of 36 valence electrons in XeF4. Because the central Xenon atom has two lone pairs of valence electrons of a molecule, the molecular geometry will help with the repulsion. The two pairs, which are nonbonding pairs, will be in a perpendicular plane to maintain minimal repulsion.
In addition to this, the four Fluorine atoms will form a square planar. Since XeF4 has an electronic geometry of octahedral, the shape of the structure is symmetrical, making the XeF4 a nonpolar molecule.
Electronegativity
The force exerted by atoms upon chemical bonding is called electronegativity. When a bond occurs, all atoms hold their electronegativity. With this, some may have higher or lower electronegativity.
When an atom has higher electronegativity, it can attract an electron pair from the bonding partners. The attraction also creates uneven sharing of electrons. Due to the electronegativity difference of two lone pairs, XeF4 forms polar covalent bonds with valence electrons. According to Pauli Scale, the Xe-F bond is polar because the electronegativity difference of the chemical equation is 1.4, but individual Xe – F bonds have zero dipoles. With this, the molecular polarity of XeF4 is nonpolar.
Dipole Moment
The dipole moment is one indicator if the XeF4 is a polar or nonpolar molecule. To calculate the dipole moment of the molecules, multiply the induced charge by the distance of separation of the atom. A polar molecule has a net dipole moment like Xenon Trioxide, but a nonpolar molecule like Xenon Tetrafluoride does not have a dipole moment.
Even though the bonds between the two atoms are polar, XeF4 is considered a nonpolar molecule. The Xe-F bonds are mutually in position with each other, which creates the vector sum of dipole moment zero. With this, XeF4 proves to be a nonpolar molecule.
Charge Build Up
If there is an accumulation of negative charges at the end of a molecule and positive charges opposite, the molecules are polar. The charge build-up results in an asymmetrical structure making the molecules polar. Since XeF4 molecular geometry has a symmetrical structure due to the bond formation and dipole moments, the molecules are nonpolar.
Regardless of electronegativity, the formal charge of XeF4 is zero. It can be determined by assuming that the bonds are covalent, ideally even sharing the electrons.
Is Xenon Tetrafluoride Polar or NonPolar?
XeF4 is nonpolar. Using the Lewis Structure, we can identify the molecular geometry of XeF4. The lone pairs of electrons and the bond angles of all the atoms created nonpolar molecules in noble gases like Xe. The F-Xe-F bond angle is 90 degrees, and it creates a square planar shape.
In Chemistry, when XeF4 is under high pressure and temperature, XeF4 reaches the melting point. It then converts a silicone component into a simple gaseous compound where the metal impurities dissolve. XeF4 reacts with water at 80 Celcius to give hydrogen fluoride, oxygen, and the noble gas Xenon.
References:
- https://www.reference.com/science/xef4-polar-nonpolar-molecule-4ff648d05ce0f916