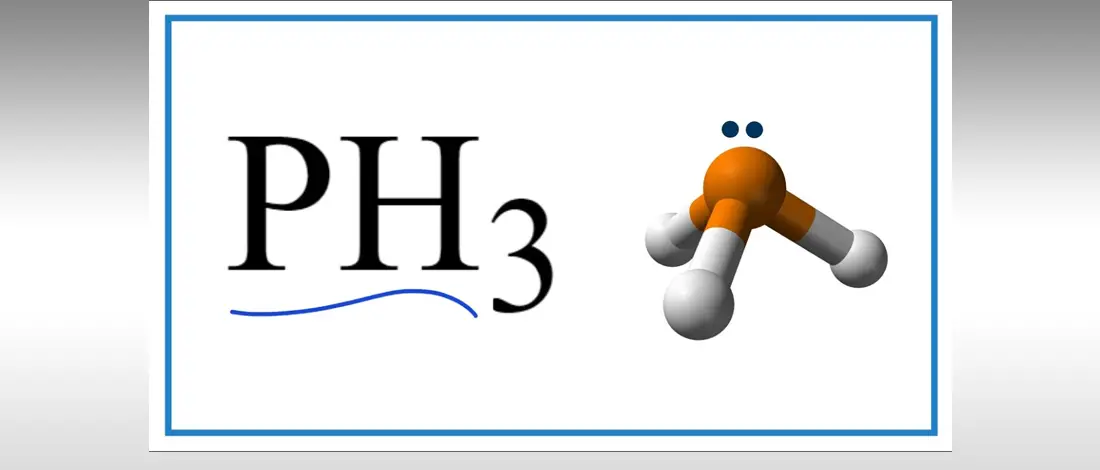
PH3 is one of the most misunderstood chemical compounds due to its polarity properties that can be polar on nonpolar. Phosphine with the chemical formula PH3 is a clear, flammable gas and highly toxic compound.
Is PH3 polar or nonpolar?
Let’s go and dive deeper into PH3 to stop the confusion.
Table of Contents
Polarity or Nonpolarity of Phosphine or Phosphorus Trihydrate
PH3 is a polar molecule because it has a bent structure due to lone pairs of electrons and electron-electron repulsion. Phosphorus’s electronegativity is a nonpolar molecule because it is the same, but since Phosphorus has a lone pair, PH3 is a polar molecule.
PH3 has five valence electrons and three hydrogens in Phosphorus, where the Hydrogen atom gives three electrons to the central atom P to meet the octet rule. The two valence electrons will form a negative region in pure P orbitals, while H will form a positive region. Due to uneven charge distribution, the lone pair gives a strong dipole moment of 0.58D, making the compound polar.
Molecular Structure & Formation
Use the Lewis Structure to identify the molecular geometry of Phosphorus Trihydrate. The central atom belongs to the P atom with a lone pair of electrons and three Hydrogen below. The trigonal pyramidal shape makes the whole molecule polar.
Hydrogen and Phosphorus are not in the same group at the periodic table. PH3 has five valence electrons and three hydrogen atoms. The molecular geometry was produced due to the one lone pair of electrons, and note that this pair avoids PH3 getting hybridized
How to Determine PH3 Polarity
Geometrical Shape
Using the Lewis Structure, the molecular geometry result is due to one lone pair of PH3 remaining at a distance from the three bond pairs. The shape of PH3 is trigonal pyramidal. Trigonal pyramidal determines that the molecule PH3 is an asymmetrical structure that does not cancel out the net charge. The geometry of these molecules is not symmetrical.
Electronegativity
Phosphine or Phosphorus Trihydrate is one best candidate for being polar molecules that have nonpolar bonds. In one lone pair orbital with three hydrogen bonds, Hydrogen atoms and a Phosphorus atom have the same electronegativity.
The electronegative property of PH3 found in the periodic table attracts shared pairs of valence electrons, creating covalent bonds. However, due to the unbonded electron, there will be asymmetrical charge distribution. With this, the PH3 is a polar molecule with nonpolar covalent bonds, not polar bonds.
Dipole Moment
It is essential to identify the dipole moment of the compound. As the compound shares electrons unequally, a dipole moment occurs. When a compound creates Dipole moments, it occurs when there is a separation of charge. When atoms form polar bonds, the difference in electronegativity gives dipole. The more significant the electronegativity difference produces, the larger the moments. The distance also indicates the size of the moment, which is the same reason the compound is polar.
In PH3’s case, the dipole moment produced is from the unshared pair that produces 0.58D, which is considered polar.
How is PH3 Used Commercially

PH3 is a clear gas that produces a rotten fish smell [1] due to substituted Phosphine and Diposphane. PH3 is used in the production of semiconductor and plastic industries. Phosphine is an essential ingredient in manufacturing LED lights. It is used in semiconductors to introduce Phosphorus to silicon crystals and dopants for creating semiconductors. In the production of flame retardants, the flammable properties of PH3 are of great use.
The toxic properties of PH3, although very harmful to humans in a higher period of exposure. White Phosphorus is used in the chemical processing of insecticide for grains, animal feeds, and leaf-stored products. In addition, PH3 is used as a polymerization initiator and fumigant that creates high risk when inhaled. PH3 is industrially made from white Phosphorus producing liquified ga
FAQS
PH3 is a polar molecule. The geometric structure is an indicator of the compound’s polarities. Using the Lewis structure, we identified that PH3 creates a nonsymmetrical shape with a 93.5 bond angle. Due to the unbonded pair of electrons, PH3 is considered a polar molecule that creates an electronegativity difference between Hydrogen’s electrons and Phosphorus.
PH3 is basic. In Lewis Theory, the basic donate pairs of electrons while the acidic accepts it. Since PH3 has unshared pairs that can be donated, PH3 is proven to be basic.
The PH3 is formed when hot boiling water or any strong base is combined. The boiling point of PH3 is -87.7 °C, and the melting point is -132.8 °C. It is more soluble in water with 31.2 mg/100 ml solubility.
In Summary
PH3 charges on its both positive and negative regions. The covalent bond has electronegative and produces dipole moments. Using the Lewis Structure, we can identify the shape of the molecule. With this, PH3 is polar even though the bond is a nonpolar covalent.
Thank you for reading this far! I hope that the information provided in this article will be helpful to you.
References:
- https://pubchem.ncbi.nlm.nih.gov/compound/Phosphine