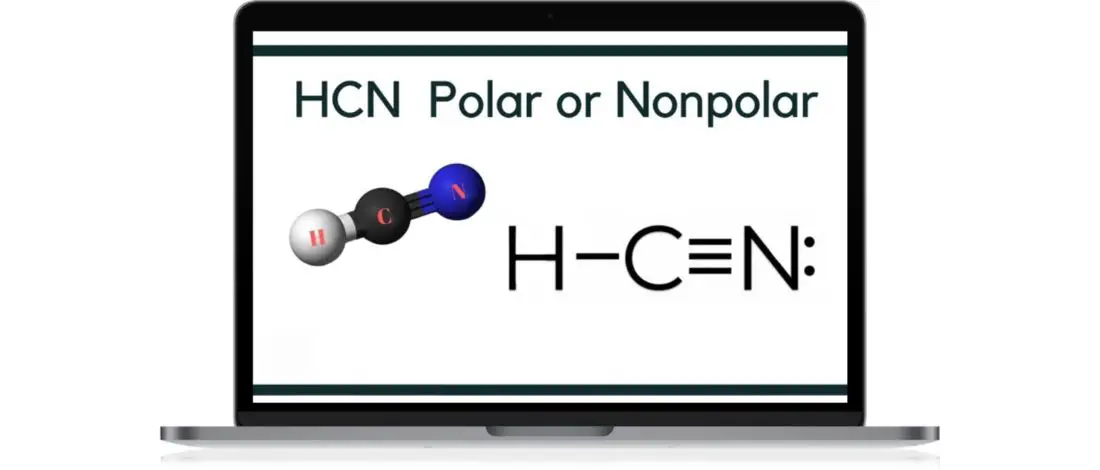
Molecular geometry has become one of the most popular topics among scientists and science enthusiasts alike. The urge to discover and study the shape and characteristics of molecules and acids has sparked topics like HCN: Polar or Nonpolar?
If you are interested in knowing more about this topic, feel free to check out this article.
Table of Contents
Polarity or Nonpolarity of Hydrogen Cyanide
HCN, or hydrogen cyanide, is a polar molecule because there is a large electronegative difference between the N and H across the linear molecule. It consists of two polar bonds whose polarities line up in the same direction.
Thus conferring an overall partial positive charge on one end of the molecule and a partial negative on the other end. [1]
The Lewis structure determines whether an entire molecule is polar or essentially nonpolar. Studies show that nonpolar molecules/nonpolar covalent bonds are symmetric. Meanwhile, polar molecules are asymmetric because they contain lone pairs of electrons on a central atom.
Application of HCN
The Hydrogen Cyanide or HCN is most commonly used in the groundwork of acrylonitrile. It is used to manufacture acrylic fibers and synthetic rubbers. The HCN has ten valence electrons and is also used to produce plastics.
Hydrogen’s electronegativity partially ionizes the cyanide anion. It is reasonably useful for various chemical reactions such as hardening steel and iron and electroplating.
What Are Its Properties?
Hydrogen Cyanide or HCN becomes a colorless liquid when placed at room temperature with an oily odor. However, it may become flammable and poisonous due to the instability of the triple bond. HCN is acidic and has a melting point of −13.29 °C or 8.08 °F. On the other hand, it has a boiling point of 26 °C or 79 °F.
Its acidity is 9.21 PKA, and it has ten valence electrons. However, when exposed to a temperature of 25 °C, its vapor pressure becomes 100 kPa. According to Molecular Geometry, the HCN features a polarity bond of 2.98 D and has a linear molecular shape.
Factors That Determine HCN Polarity

Geometrical Shape
Various factors could determine the polarity of a substance. To further discuss the molecular geometry of molecules, the geometrical shape of the molecule may be characterized as asymmetric or distorted.
It contains various electronegativities bonded, and either of its ends may be slightly positive and negative. On the contrary, symmetrically shaped molecules have identically bonded elements without any unshared pairs of electrons.
Electronegativity
Electronegativity is a measure of an atom’s aptitude to attract its shared electrons. According to the periodic table, electronegativity values increase as the atom moves from left to right across a period. At the same time, it also decreases as it moves down a group.
In the Lewis dot structure of the three electron pairs of HCN, the nitrogen atom has a large electronegativity difference from the carbon atom. The hydrogen atom is becoming the negative pole. The bond formation between these atoms becomes a polar covalent bond.
Dipole Moment
This occurs when there is a separation of charge between two atoms/compounds formed in a covalent. It arises from the difference in electronegativity.
It is also the product of charge on atoms and the distance between negative and positive charge centers. Therefore, 2.98 Debye is the dipole of the HCN molecule according to the Lewis dot structure.
FAQS
HCN with ten valence electrons is a molecular bond with a linear-shaped molecule, also known as a covalent bond. It involves the sharing of electron pairs or bonding pairs resulting in a non-zero dipole.
HCN is a linear-shaped polar molecule with a single bond between H and N between the central C atom. This chemical bond has a permanent dipole moment. Since HCN contains N, which is not directly bonded to hydrogen atoms, it has dipole-dipole forces act between the electron pair of the HCN molecule.
Key Takeaways
Based on round-the-clock research, HCN is a polar bond molecule due to the large electronegativity difference across the linear molecule. It has a polarity bond of 2.98 D, ten valence electrons. It may become poisonous due to the instability of the triple bond. Since the HCN consists of two linear molecule polar bonds formed, it confers an overall partial negative charge and a slightly negative charge on both ends.
Also, as the Lewis structure suggests, the HCN is asymmetric in molecular shifting toward the more electronegative atom, which has a partial negative charge. It also contains various electronegativities bonded, making the chemical bond polar molecule, like the two hydrogen atoms and Oxygen atom, which has a net dipole moment making.
Thank you for reading this far! I hope that the information provided in this article will be helpful to you.
References:
- https://www.reference.com/science/hcn-polar-non-polar-f4c06eb4802acc63