The simplest kind of alcohol is methanol, which has a methyl group connected to the hydroxyl group. Methanol is colorless and has an odor that is comparable to ethanol. It is explosive and light. Additionally, it is a polar solvent that is occasionally referred to as wood alcohol due to its origins in wood distillation.
And to answer is CH3OH polar or nonpolar molecule, we gathered below the details about the polarity of this element.
Table of Contents
CH3OH: Is It Polar Or Non-polar?
CH3OH or methanol is a polar molecule. Methanol is a polar molecule because the OH group dominates, and oxygen is more electronegative than carbon and hydrogen atoms, making it polar. As a result, oxygen is partially negatively charged, while carbon and hydrogen are positively charged.
CH3OH cannot be non-polar due to the charge difference between the atoms in the methanol. Because of the two lone pairs of electrons, oxygen atoms have a higher electron density.
These pairs result in a net dipole pointing toward the Oxygen atom, making methanol a polar molecule. Apart from the difference in electronegativity, the CH3OH has an asymmetric shape, which eliminates the possibility of non-polarity. [1]
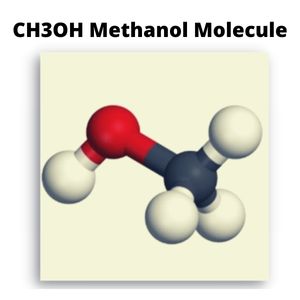
Understanding Its Structure

Polarity
Methanol is polar because of the dominance of the OH group, which is polar because oxygen is more electronegative than the other atoms. As a result, O acquires a negative fractional charge, whereas carbon and hydrogen acquire a positive charge.
Bond Angles
In the chemical, the core Carbon atom forms four bonds, three with a Hydrogen atom and one with the hydroxyl group. Because this carbon atom is sp3 hybridized and forms a tetrahedron, it possesses a 109.5-degree bond angle, including its bonding atoms.
On the other hand, O forms a one sigma bond with two lone pairs, resulting in a bond angle bend caused by bound pair-lone pair repulsion forces. The bond angle is minimized to 104.5° as a result of this.
Hybridization
The oxygen has four sp3 hybrid orbitals since it is sp3 hybridized.
The O-H sigma bonds are molded when one of the sp3 hybridized orbitals collides with a hydrogen’s s orbitals.
The C-O sigma bond is created when an sp3 hybridized orbital collides with an sp3 hybridized orbital from carbon.
The remaining sp3 hybridized orbital contains both sets of lone pair electrons on the oxygen.
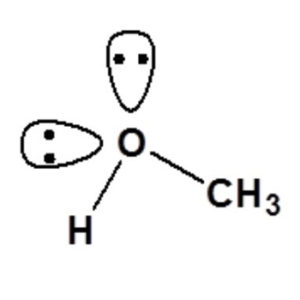
Molecular Geometry
When the Lewis structure of CH3OH is analyzed, it does not appear to be a symmetrical molecule. However, to evaluate if CH3OH is polar, we must analyze the molecular geometry. There is unequal sharing of electrons and a net dipole in CH3OH. CH3OH- is thus a polar molecule.
Molar Mass
Methanol is a polar solvent that is occasionally referred to as wood alcohol due to its origins in wood distillation.
Methanol has a molecular mass of 32.04 g/mol.
It has a boiling point of 64.7°, and its melting point is −97.6 °.
Due to its low melting point, CH3OH is also used as a gasoline additive.
Additionally, it is utilized in the production of acetic acid and formaldehyde.

Acid or Base
Methanol is a weak acid. Hence its conjugate base is very strong. It is alkyl alcohol, a volatile organic compound, and a primary alcohol. It is the conjugate acid of methoxide.
Methanol is the simplest alcohol, containing one carbon, three hydrogen bonds, and one hydroxyl group. Additionally, this is referred to as methyl alcohol.
Related Posts:
Factors Affecting CH3OH Polarity

Dipole moment
One of the factors in determining the polarity of a molecule is its dipole moments. The polarity of a molecule is identified by its dipole moment. Additionally, the dipole moment is related to a molecule’s polarity. The stronger the molecule’s dipole moment, the more polar it is.
Geometrical Shape
The molecules that have symmetrical geometrical structures are considered nonpolar molecules in nature. If any polar bonds are present inside the molecule, they are canceled by each other, making the whole molecule nonpolar.
The molecules with asymmetric structure and polar bonds inside them result in a net dipole moment and make them polar.
Electronegativity
Polar molecules are molecules where atoms share an unequal proportion of bonded electrons. The electronegativity of an atom determines its capacity to attract bonding electrons. [2]
The more electronegative atom draws the bonded atoms slightly toward a molecule with two atoms. It obtains a partial negative charge, while the other atom gains a partial positive charge. Also, a chemical is polar if a charge separation happens on it.
Geometrical Structure
The central atom is unpaired and forms four sigma bonds with three hydrogen atoms and one hydroxyl group. When carbon is considered the center, the form of the methanol molecule is tetrahedral. Oxygen forms a bent shape by forming two sigma bonds with two lone pairs attached. The angle of the bond is approximately 104.5 degrees.
Uses of H2S
- Sulfur and sulfuric acid are produced as a result of this process. [1]
- Pesticides, leather, and medications are all made with this chemical.
- It is also utilized in nuclear power facilities to manufacture heavy water, a byproduct of nuclear power.
- H2S is a chemical compound that is utilized in chemical analysis.
- It can also be used as a disinfectant in agricultural settings.
- It is the primary source of elemental Sulfur.
- It is required to function in signaling pathways in the human body properly.
FAQS
No, CH3OH is an asymmetrical molecule. The form of the methanol (CH3OH) is asymmetrical. A molecule’s symmetry plays a significant role in defining its polarity. The ionic bond is formed among charged atoms. Some examples of ionic molecules are KCl, NaCl, etc.
The O-H bond is the most polar in the methanol molecule. The oxygen atom has a greater electronegative charge than the carbon and hydrogen atoms. As a result, O gains a partial negative charge, resulting in a polar bond.
Key Takeaways
Due to the uneven charge distribution of the atoms and the asymmetric molecular structure of methanol, it is polar. CH3OH is a polar molecule because its asymmetric structure does not eliminate the dipole-dipole moment. Additionally, the molecule generates a net dipole moment. Methanol contains covalent bonds, and polar or nonpolar covalent bonds are possible.
When two atoms create a covalent bond, polar molecules are formed when the charge distributions of the atoms are uneven. In contrast, nonpolar molecules have an identical charge distribution, making a covalent connection between both atoms. The exchange of electrons between atoms occurs when two or more atoms create a chemical bond to form a molecule.
Thank you for reading this far! I hope that the information provided in this article will be helpful to you.
References:
- https://www.sciencedirect.com/topics/chemistry/polar-covalent-bond
- https://www.thoughtco.com/examples-of-polar-and-nonpolar-molecules-608516