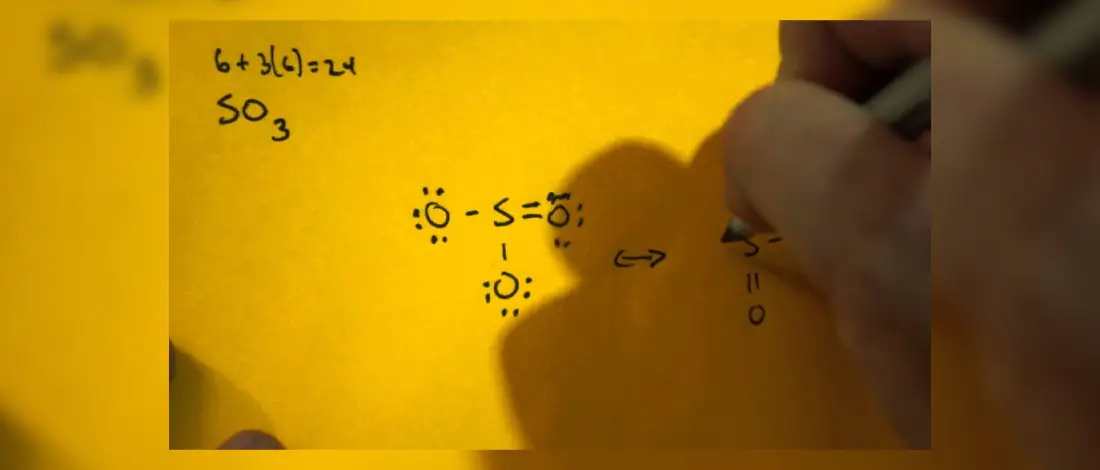SO3 is the formula for the chemical compound Sulfur Trioxide. This chemical compound plays a vital role in producing sulfuric acid for industrial purposes.
But, SO3 is the primary contributor to acid rain in the atmosphere and is a form of pollution. So, let’s dive deep into the subject and know the SO3 Lewis structure, its molecular geometry, polarization, and hybridization.
Table of Contents
Understanding the Lewis Structure of SO3
First of all, the Lewis Structure, also known as the electron dot structure, determines the number of valence electrons present in an atom.
It also shows how these electrons play a role in the bond formation process, which allows the formation of a molecule like SO3 (a trigonal planar molecule). This structure illustrates the number of bond electron pairs and valence electrons within the Sulfur Trioxide molecule through a certain geometric manner.
Moreover, the Lewis structure helps in understanding the number and nature of a bond through lines. In fact, this structure is an important component for anybody studying atomic chemistry.
Its Properties & Structure

The structure of SO3 is trigonal pyramidal. But, can you enumerate its different properties and structures? So, let me explain them to you.
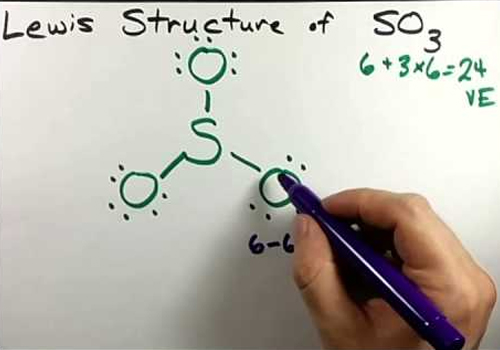
SO3 Lewis Structure
The sulfur trioxide SO3 is a tetra-atomic chemical molecule where three oxygen molecules and a sulfur bond have the same number of valence electrons.
There are three double bonds around a sulfur atom with oxygen atoms in a SO (Sulfur Monoxide) molecule. Each of these bonds has two lone pairs in the SO3 Lewis structure. However, there is no one lone pair on the sulfur atom in the lewis structure of the SO2 (Sulfur Dioxide).
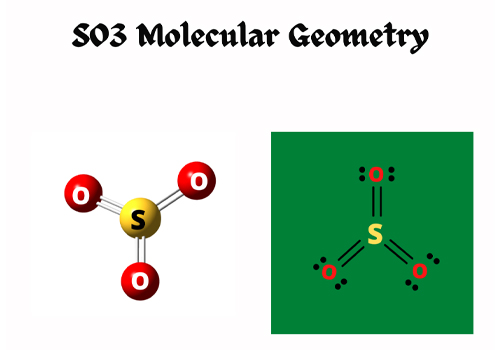
SO3 Molecular Geometry
The molecular geometry of an SO3 molecule ion can be predicted by using the molecular hybridization and the Valence Shell Electron Pair Repulsion Theory (VSEPR Theory), and the molecular hybridization theory.
The molecular geometry of Sulfur Trioxide SO3 is trigonal planar as the three S O bond is a nearly double bond tilted at a 120-degree bond angle with an electronegativity difference between a central sulfur and oxygen atoms.
This difference allows for a greater electron cloud produced by the presence of both sulfur atom and oxygen atom.
SO3 Hybridization
Single shared double covalent bonds have double bonds – one sigma bond and one pi bond. So, in a single SO3 molecule, the total number of sigma bonds is three, and the total number of lone pairs is zero.
All atoms possess sp2 hybridization, and oxygen atom have two lone pairs and one sigma bond. As a result, the hybridization of oxygen atoms must be sp2. Then for one sulfur atom, it has no lone pair but has three sigma bonds, and its hybridization is also sp2.
Related Posts:
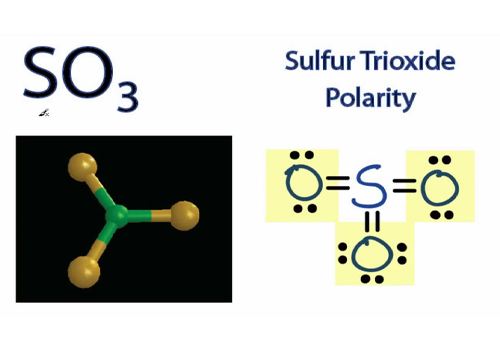
SO3 Polarity
Are you confused about whether SO3 is polar or non-polar?
Then, let me explain to you in the simplest way.
SO3 is non-polar. All oxygen atoms are symmetrical, having the same number of lone pair electrons and bonds.
If we use the VSEPR Theory, we can conclude that as all oxygen spreads out, they repulse each other, which shows SO3 as non-polar.
As SO3 is trigonal planar and has no positive and negative charges, it has equal charge distribution on the center atom.
Steps In Drawing SO3 Lewis Structure
Learning how to draw SO3 Lewis structures is vital to every chemistry student.
The key to learning it is to understand the practice and steps.
- Find the total valence electron for the SO3 molecule. You can use the periodic table of elements.
- Place the least electronegative atom in the center. Remember that H always goes outside.
- Place two valence electrons in between atoms to form a chemical bond.
- Follow the octet rule, complete octets outside atoms. Note that H only needs two valence electrons.
- Based on the octet rule, move the valence electron from outer atoms if the center atom does not have an octet to form double to triple bonds.
Additional steps:
- If there are extra electrons after following the steps above, you can add them to the central atom. Note that elements in Period Three (or Third Row Elements) can have more than six valence electrons.
Lastly, check the Formal Charges to ensure you have the perfect Lewis Structure [1].
FAQS
Primarily, SO3 has three structures, but when you set it out completely, there are seven resonance structures altogether.
There are three bonds of electrons in the SO3 chemical structure, where three double bonds contain four electrons each.
Key Takeaways
Sulfur Trioxide is one of the Period Three elements, which accommodates more than six electrons. It’s fascinating to know that SO3’s molecular behavior is not as extraordinary as other elements that have the same components.
As we discussed SO3, its Lewis structure, molecular geometry, hybridization, and polarity, for sure, you can now create a molecular structure with ease. So cheers to that!
Thank you for reading this far! I hope that the information provided in this article will be helpful to you.
Reference:
- https://terpconnect.umd.edu/~wbreslyn/chemistry/Lewis-Structures/lewis-structure-for-SO3.html