The hydrogen sulfide chemical formula is H2S. It’s toxic and explosive, with the smell like rotten eggs. Additionally, it endangers the ecology.
For more details about hydrogen sulfide, our group gathered below some interesting facts, including the H2S Lewis structure, molecular geometry, and the guide on how to draw the Lewis structure of H2S.
Table of Contents
Understanding the H2S Lewis Structure
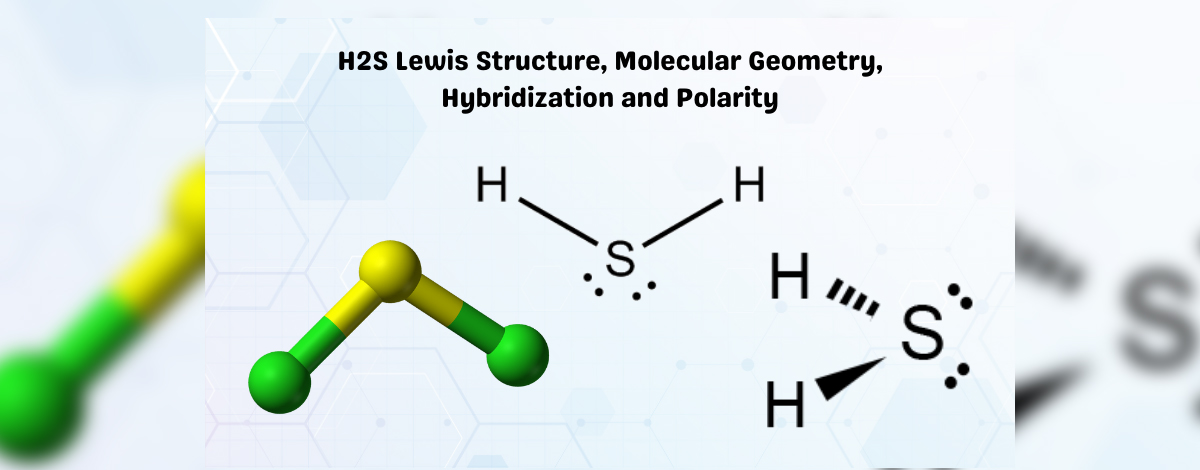
Hydrogen Sulfide’s Lewis Structure is simple to draw and comprehend. Both hydrogen atoms in this combination require one valence electron to form a covalent bond with Sulfur. To meet the condition for the octet rule, Sulfur requires eight electrons. However, because Hydrogen is a Group 1 element, it only needs one electron to make hydrogen stable.
Arrange the valence electrons of the Sulfur around it in the center. Now, place two Hydrogen atoms on each side of the central atom. By contributing an electron to both Hydrogen atoms, the valence electrons of Sulfur are utilized to make Hydrogen persistent.
Since the remaining four electrons are lone pairs of electrons, they appear as dots near the Sulfur atom. This combination has H atoms with a complete valence shell and two non-bonding electrons.
Closer Look at the Structure
Hybridization
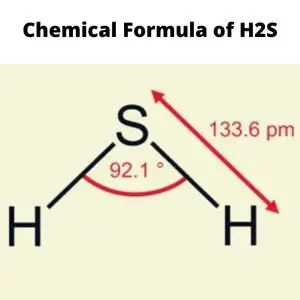
The central Sulfur atom is in the middle, along with the two hydrogen atoms forming the bond angle of 92.1 degrees or less than 180. Calculate the number of atoms that are connected to the central atom. Second, count the number of lone pairs on that central atom.
So, there are four lone pair electrons around Sulfur, implying that Sulfur has two lone pairs. Finally, add the number of attached atoms to the central atom + the number of lone pairs of that central atom. As a result, four indicates that H2S hybridization is Sp3.
Valence Electrons
In the compound, there are two hydrogen atoms and one sulfur atom. Sum the valence electrons of both the Hydrogen and Sulfur atoms to get the total number of valence electrons in Hydrogen Sulfide.
Each hydrogen atom has only one electron. As a result, the Hydrogen atom has two valence electrons, two atoms, while the Sulfur atom has six. As a result, H2S has a total of eight valence electrons.
Molecular Geometry
Because the two lone pair electrons on the sulfur core atom oppose each other and nearby bonded pair electrons, the molecular geometry of H2S is bent.
As a result, these pairs take the least repulsion location and achieve stability.
Thus, the final form or molecular geometry of H2S resembles a V-shaped or bent structure.
Bond Angle
A bond angle of fewer than 180 degrees exists between two sulfur atoms in the central atom of the h2s molecule. The molecule bends due to two unbonded electron pairs. Due to the electronegativity values of Hydrogen and Sulfur, it is considered a slightly polar molecule.
Acid or Base?
Hydrogen sulfide has the chemical formula H2S.
The formula indicates that it is composed of two hydrogens and one sulfide atom.
Slightly denser than air, it has the potential to be extremely explosive.
Hydrogen sulfide burns blue when combined with oxygen, forming sulfur dioxide and water.
t is water-soluble and functions as a weak acid.
Polarity
Hydrogen Sulfide is a somewhat polar molecule due to its bent geometric structure and the tiny difference in their electronegativity value between Hydrogen and Sulfur, which results in a non-zero net dipole moment.
Molar Mass
H2S has a density of 1.363 g dm-3 and a molar mass of 34.08 g/mol. H2S has a melting and boiling point of -82°C and -60°C, respectively. The covalent bond formation in H2S is formed when the sulfur atom completes its octet by sharing two electrons with two hydrogen atoms.
Related Posts:
How to Draw H2S Lewis Structure

Here are the following ways on how to draw an H2S Lewis structure.
- Determine how many valence electrons H2S has. Sulfur is in the 16th group of the periodic table and hence has six valence electrons, while Hydrogen has two.
- Next, figure out the central atom. Draw a skeleton structure of H2S using just single bonds. Remember that Hydrogen requires a single electron to complete its valence shells.
- Distribute electron pairs among the atoms. The central sulfur atom transfers electrons to both nearby hydrogens. In creating a bond, two out of six valence electrons participate.
- Distribute the remaining electrons among the remaining atoms. The outer shell of Hydrogen is already full because it shares two electrons with a single bond. So, no need to put electrons around the H atom.
- Finally, reduce the charges on the H2S atom by converting lone pairs to covalent bonds.
Uses of H2S
- Sulfur and sulfuric acid are produced as a result of this process. [1]
- Pesticides, leather, and medications are all made with this chemical.
- It is also utilized in nuclear power facilities to manufacture heavy water, a byproduct of nuclear power.
- H2S is a chemical compound that is utilized in chemical analysis.
- It can also be used as a disinfectant in agricultural settings.
- It is the primary source of elemental Sulfur.
- It is required to function in signaling pathways in the human body properly.
FAQS
H2S has two lone pairs and two bound pairs, according to the Lewis structure of H2S. Four zones of electron density always form a tetrahedral electron geometry; therefore, the electron geometry of H2S is tetrahedral.
H2S has a bent molecular geometry. The repulsion causes the bond pairs to alter form from straight to curved. Electron geometry considers the electrons and unshared pairs for determining the shape of H2S, whereas molecular geometry examines just the molecules’ linked atoms.
The type of intermolecular force that the H2s molecule is dipole-dipole force. According to VSEPR theory, this indicates that the molecule’s structure is asymmetrical, and as a result of its asymmetrical form, the molecule is slightly polar. Polar molecules participate in intermolecular interactions called dipole-dipole forces. [2]
In Conclusion
To conclude, these are the key takeaways. Hydrogens make a single bond with the sulfur atom in the H2S lewis structure. The total valence electron of H2S is 8 with a bond angle of 180 degrees. H2S is a polar molecule with curved molecular geometry.
The Lewis or lewis dot structure plays an important role in determining a compound’s electron and molecular geometry. According to the Lewis structure of H2S, two hydrogen atoms are located on either side of the center sulfur.
Thank you for reading this far! I hope that the information provided in this article will be helpful to you.
References:
- https://sciencing.com/uses-hydrogen-sulfide-5515916.html
- https://brocku.ca/student-life-success/wp-content/uploads/sites/220/Learning-Services-Student-Resources-Intermolecular-Force.pdf


