Water has a basic Lewis structure, making it one of the most basic chemical substances to comprehend.
And now, to further know more interesting information about H2O and its electronic geometry, Lewis structure, and others, our team did some research about the structure of H2O.
Table of Contents
Understanding the Electronic Geometry of H2O
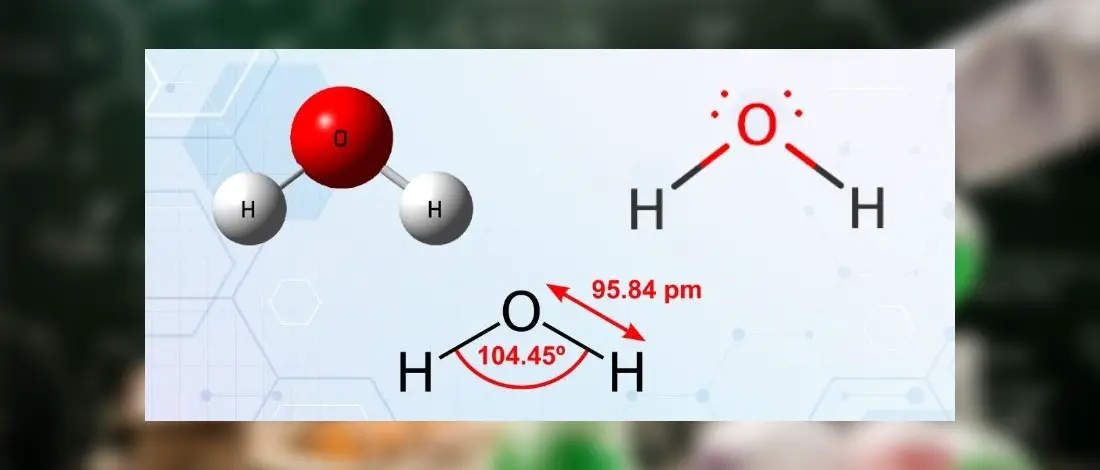
The H2O molecule is composed of two hydrogen atoms and one oxygen atom. It forms a bond angle of 104.5°. As a result, it is feasible to determine that it is bent in the form of an H2O molecule.
According to Lewis’s structure, a lone pair exists when all of the atom’s valence electrons are unpaired.
The H2O molecule’s Lewis structure reveals two solitary sigma bonds between the O and H. Additionally, and these connections leave two lone pairs of electrons on the oxygen atom, which adds significantly to the H2O molecule’s tetrahedral bent geometrical configuration.
Closer Look at the H2O Structure
Electronic Geometry
Water is classified as tetrahedral in terms of electron-group geometry with four electron groups. The four electron groups are formed by the two solitary hydrogen bonds and the lone pairs of Oxygen. Due to lone pairs in water, its molecular structure is twisted. [1]
Molecular Geometry/Shape
Any molecule’s molecular geometry is determined by its Lewis structure. Oxygen creates two single sigma bonds with H atoms in the H2O molecule. Then, The two lone pairs of electrons push these Hydrogen atoms on the Oxygen atom.
Due to the repulsion forces of lone pairs being greater than those of bonded pairs, the arrangement of atoms is deformed. As a result, the water molecule’s molecular geometry is angular or v-shaped.
Lewis Structure
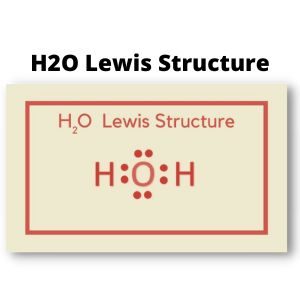
In contrast to Hydrogen atoms, Oxygen atoms will always occupy the central atom. Put O in the middle, with H on either side. It takes one more electron to stabilize each of these Hydrogen atoms. Oxygen also requires two valence electrons to complete its octet rule.
Both Hydrogen atoms will share one valence electron to produce a stable structure. Show a single bond on both sides to demonstrate electron sharing. O and H molecules are solitary bonds in the Lewis structure of H2O.
Thus, this molecule has two lone pairs and two bonding pairs. The Lewis structure of H2O is drawn to achieve each atom’s insufficiency.
Valence Electrons
The H2O molecule has an overall valence electrons count of eight valence electrons. This result was obtained by multiplying the total number of valence electrons in this molecule by the total valence electrons in the Hydrogen and Oxygen atoms.
The total number of valence electrons in a hydrogen atom is two valence electrons since there are two hydrogen atoms. By contrast, the oxygen atom forms a total of six valence electrons. As a result, the H2O molecule has a total number of eight valence electrons.
Bond Angle
For molecules with a tetrahedral geometry, the bond angle is 109°; however, when the molecular geometry of water is distorted owing to the existence of lone pairs of electrons, the bond angle decreases from 109° to 104.5°, indicating that the molecule is deformed.
Dipole Moments
Dipole moments occur when one atom is more electronegative than another, resulting in that atom tugging more firmly on the shared pair of electrons, or when one atom has a lone pair of electrons and the difference of electronegativity. [2]
One of the most common examples is the water molecule, one oxygen atom, and two hydrogen atoms.
The differences in electronegativity and lone electrons give Oxygen a partial negative charge and each hydrogen atom a partial positive charge.
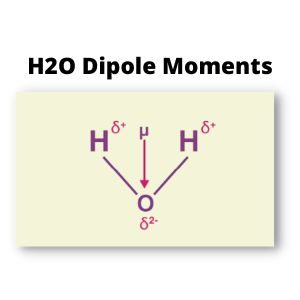
Polarity
Water is a polar molecule. Due to the lone pair, H2O results in a bent-shaped molecule as per VSEPR theory so that the vectors representing each bond’s dipole moment do not cancel out. They are making the water a polar molecule.
Hybridization
Each O and H atom in a water molecule has a sigma bond and no pi bonds. As previously stated, sigma bonds are the strongest type of covalent bond. When two atoms share electrons and establish bonds, hybridized orbitals arise. These orbitals aid in the prediction of the molecule’s hybridization. Here, we shall examine the hybridization of the Oxygen atom, which shares two electrons to complete its outer shell from its valence electrons with both Hydrogen atoms.
Three 2p and one 2s orbital of Oxygen are hybridized due to lone pairs and two pairs of bonding electrons. And because four of Oxygen’s orbitals are hybridized, H2O’s hybridization is sp3.
Related Posts:

Electron-Group Geometry
The H2O molecule possesses four electron groups. Hence it is classified as tetrahedral in terms of electron-group geometry. The two single bonds to Hydrogen and the lone pairs of Oxygen constitute the four-electron groups. Water’s molecular form is bent because it has lone pairs. According to the VSEPR hypothesis, electrons try to reduce repulsion. Therefore the lone pairs are close to one another.
Molecular Geometry
The atomic number of Oxygen is 8. It possesses six valence electrons and requires more electrons for the octet rule. The atomic number of a hydrogen atom is one, and it takes one valence electron to create a duet configuration.
Therefore, two hydrogen atoms create one single bond with the center oxygen atom. The valence shell of the oxygen atom has two electron pairs. Therefore, according to the VSEPR hypothesis, water has a v-shaped or bent shape.
FAQS
Any molecule’s molecular geometry is determined by its Lewis structure. Due to the repulsive forces between lone pairs, the molecular shape and form of the water are bent.
In water, the oxygen atom has two lone pairs. When the bond angle of the H-O-H bond is 104.5°, these two lone pairs reject the H-O bonded pairs so strongly that the molecule is in its lowest energy configuration. As a result, the water molecule is non-linear.
Water is a polar molecule. Due to the unequal distribution of electrons among the atoms and the asymmetrical form of the molecule, water molecules have two poles – a positive electric charge on the pole side of H and a negative charge on the pole side of Oxygen.
In Summary
Water is one of the elements that have a basic Lewis structure. Lewis structure helps determine the bond produced and electrons in the bond formation. The bonding pair of electrons are the electrons involved in the bond formation. Nonbonding electron pairs are those that do not participate in any bond formation.
Thank you for reading this far! I hope that the information provided in this article will be helpful to you.
References:
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Chemical_Bonding/Lewis_Theory_of_Bonding/Geometry_of_Molecules
- https://study.com/academy/lesson/dipoles-dipole-moments-molecule-polarity.html