Molecular geometry and electron geometry in chemistry are how atoms are arranged around a center in a three-dimensional space. This arrangement provides the familiar molecular shape and bond angles to atoms.
But, how is the molecular geometry different from the electron pair geometry? Learn from our thorough discussion below.
Table of Contents
What's the Main Difference Between Electron Pair Geometry & Molecular Geometry
Molecular geometry in chemistry is a concept that refers to the arrangement of atoms about the central atom in a three-dimensional space.
The definition of electron geometry is the arrangement about electron groups of atoms. The bond is 105 degrees.
If the lone pairs of electrons are situated in the molecule, it changes the molecular geometry and not the electron pairs.
When electrons are bonded to the central atom and do not have lone pairs, the electron geometry and molecular geometry are similar.
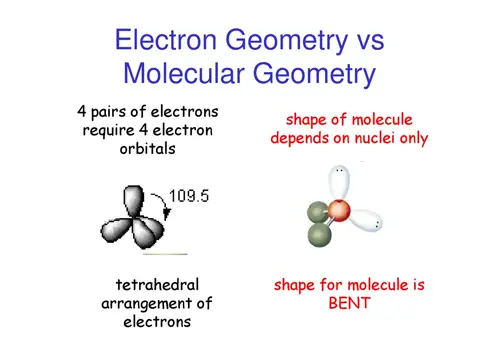
Molecular & Electron Geometry Variance
Molecular Geometry
Purpose:
Molecular geometry has concepts that allow you to understand the entire atom along with the arrangement. It is the 3D arrangement in all of the atoms.
The molecular works to help scientists in understanding the shapes of more complicated atoms. A lone pair isn’t included in the molecular geometry.
The shapes of the molecules have important roles that determine the job that these molecules are performing in our bodies.
It is dependent on electrons and number of lone pairs. Trigonal planar has a bond angle of 120 degrees.
Examples:
Let us use H2O to understand this better.
Oxygen is a central atom that has six valence electrons. The hydrogen donates a total of two electrons. In total, there are eight. In this example, there are four electron groups and two lone electron pairs. What’s more, there are two single bond pairs.
In this case, the molecular geometry is bent. We can draw the molecular formation quickly.
Another example is CO2.
Carbon dioxide is what you call a linear molecular.
Related Posts:
Electron Pair Geometry
Purpose:
In VSEPR theory, the repulsion between the electron geometry pair shapes is electron geometry. (1)
In electron geometry, it reduces the repelling energy. A lone pair of electrons gives you the most excellent repelling results due to repulsion in theory.
Electron geometry uses dots to identify the number of valence electrons included atoms have. You can count the bonding electrons and the non-bonding electrons pairs of the electron group that encircles the central atom.
The trigonal planar concept means it has three electron groups. A trigonal planar is made out of three equally spaced sp2 hybrid orbitals.
The Trigonal Bipyramidal has three sides. The Trigonal Bipyramidal has bond angles of 120 degrees.
According to VSEPR, the bond pairs are spaced around the valence shell so that it achieves the furthest distance from each other in electron geometry.
In electron geometry, only the bonding electrons pairs contribute to the final shape of the molecule. It is the difference between electron geometry and molecular.
The lone pairs of electrons do not determine it. The valence electron pairs stay away from each other in electron geometry.
Examples:
A molecule that features two bonding electron pairs and has no nonbonding pairs of electrons, like carbon dioxide, is linear.
The water and ammonia molecules have four valence shell electron groups. The water has two bonding and two nonbonding pairs of electrons, resulting in a v-shape in electron geometry.
The two hydrogen atoms are forced closer together in the account for the two pairs of the non-bonding electrons.
Ammonia, for instance, is a lone pair of electrons other than a bonding one.
FAQS
Molecular geometry is important because it helps you understand the molecular structure of a specific compound that helps determine the shape and phase of matter. It is as essential as electron geometry.
Yes, the shape in molecular and electron geometry matters as it helps in determining the properties.
Molecular Geometry vs Electron Pair Geometry - In Conclusion
Electron geometry is about the arrangement of electron groups. Molecular geometry helps us understand the number of lone pairs of electrons. In electron geometry, we determine the molecular shape. In molecular geometry, we only consider the bond electron pairs.
Electron geometry and molecular geometry are similar when all electrons are bonded and don’t have lone pairs. If lone pairs – those that are not bonded to any other atom – are located in a molecule, this relatively changes the molecular geometry, and not the electron geometry.
References:
- https://chemed.chem.purdue.edu/genchem/topicreview/bp/ch8/vsepr.html


