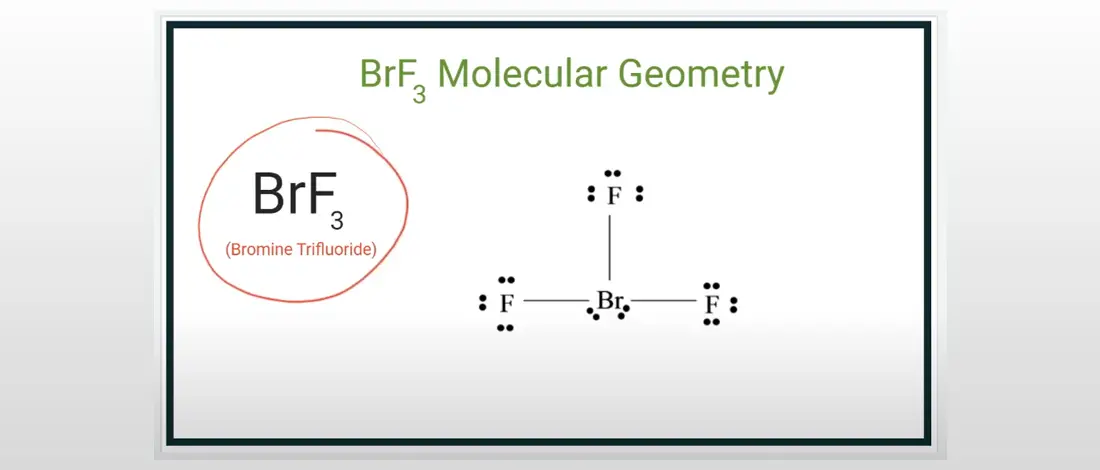
Are you curious about what a molecule like BrF3 looks like without peeking inside a microscope? In Chemistry, the chemical bonds between Bromine and Fluorine create bond formation, bond angles, and hybridization.
In this post, we will give you answers on the molecular geometry of BrF3 along with other factors to consider.
Table of Contents
Understanding the Molecular Geometry of BrF3
Bromine and Fluorine atoms are Halogens. Because of the solid interhalogen compound of the entire molecule, the hybridization of Bromine Trifluoride can be utilized as a fluorinating agent. It is in the clear to yellow liquid form and has a strong odor [1]. The chemical formula for Bromine Trifluoride is BrF3, and it reacts in different elements except for inert gases.
The outer shell of the Bromine molecule has seven valence electrons, and three of them bond with the three fluorine atoms. The BrF3 molecular geometry is in T-shaped or Trigonal Bipyramidal with Bromine as the central atom. The shape is affected because of the three bonded pairs of electrons and two lone pairs of electrons.
Other Factors to Consider

Polarity
Because of the relatively high difference of electronegativity between the bromine atom and fluorine atoms, the bonds of the BrF3 are considered polar. Because of the presence of the two lone pairs in hybridization, the molecule’s shape is bent on certain bond angles.
The single pairs are located in the triangle plane and cause uneven negative charge distribution on the central atom. Unlike a nonpolar molecule, BrF3 has an uneven distribution of the charge that affects the molecule’s geometry making it polar.
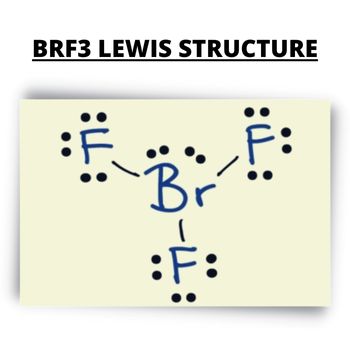
Lewis Structure
The Lewis structure or electron dot structure is a diagram that represents the single pair and bonded pair of the atoms in the molecule hybridization. The single bond is shown using lines with bond angle, and the lone pairs are presented using dots. This will help understand the distribution of the electron pair and the geometry of compounds like BrF3 and CO2.
For the Lewis Structure of the BrF3, identify how many valence electrons this compound has. The bond angle of bromine trifluoride is in a T-shaped form that is slightly smaller than 90 degrees for the lines. With Br as the central atom, place the 28 valence electrons around to achieve the octet fulfillment.
Related Posts:
- SF4 – Is It Polar or Not?
- Ch2Cl – Is It Polar or Not?
- NH3 – Is It Polar or Not?
- Molecular Geometry vs Electron Pair Geometry
- I3 Lewis Structure
Hybridization
In identifying the hybridization of bromine trifluoride, take the bromine atom and check its electron configuration and D-Orbitals. The BrF3 has seven electrons in the outermost shell for hybridization. Br and F will form bonds and will have two lone pairs and three covalent bonds.
The Br-F bonds and the hybridization value answer is 5. Fluorine does have a higher oxidative capacity, and it forces bromine to promote electrons. With this, Bromine can use the D-Orbitals for hybridization that is useful for identifying the geometry of the bond pairs.
Molecular Orbital Diagram
The molecular orbital diagram describes the location and behavior of electrons in molecules. The molecule’s physical properties are affected due to lone pairs bond formation with valence electron pairs. This function identifies the shape of hybridization by finding the electron in its specific region when these form bonds.
The MO theory deals with the energetic and spatial property of an electron pair. It also talks about the linear combination of atomic orbitals to form molecular orbitals.
FAQS
No, BrF3 is not a trigonal planar. BrF3 has single pairs at the center atom, and for a compound to be trigonal planar, the center atom should not have a lone pair. The bond angle of the BrF3 is not around 120 degrees, so technically, it is not a trigonal planar. In addition to this, trigonal planar is only bond-bond repulsion, and BrF3 is not, proving that it is a trigonal bipyramidal.
Based on VSEPR Theory, the molecular shape of BrF3 is a trigonal bipyramid. In hybridization, the two single pairs of electrons will occupy the equatorial positions. The molecules are T-shaped to minimize the repulsion between the single pairs; there will be a bent in its shape.
Final Thoughts on BrF3 Molecular Geometry
To create a perfect trigonal bipyramid, the lone pairs spreading should be greater than bonded pairs. Without looking at the microscope, we know that the chemical bonding of one Br and three F atoms produced a T-shaped or trigonal pyramidal molecule. The periodic table and other factors to consider mentioned above gave answers about the molecular geometry of the bromine trifluoride.
Thank you for reading this far! I hope that the information provided in this article will be helpful to you.
References:
- https://pubchem.ncbi.nlm.nih.gov/compound/24594