Polarity is one of the properties of a compound related to other properties such as boiling and melting point, solubility, and molecular interactions between molecules. Polar and nonpolar molecules differ significantly.
Here are the steps to help you determine if a molecule is polar or nonpolar.
Table of Contents
How Do I Know If A Molecule is Nonpolar Or Polar?
Nonpolar molecules consist of identical sides around the central atom and therefore have no unshared pairs of electrons.
The atoms in a molecule have equal or nearly equal electronegativities and have zero or very small dipole moments.
By contrast, a polar molecule consists of lone pairs of electrons on a central atom and therefore has unequal sharing of electrons. It has a permanent dipole moment, which arises from differences in electronegativities between atoms.
One part has a partial positive charge, while the other part has a partial negative charge. These two electrically charged regions are called poles.
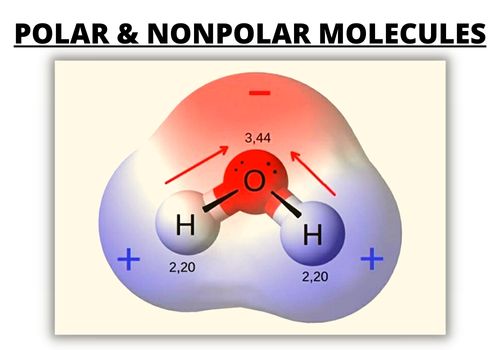
| Polar Molecules | Nonpolar Molecules | |
| Separation of Electric Charges | Has a positively charged end and a negatively charged end | No separate electric charges |
| Distribution of Electrons | Asymmetrical | Symmetrical |
| Formation | Polar molecules | Nonpolar molecules |
| Net Dipole Moment | Yes | None |
| Reactivity | Yes and forms solutions | None |
| Dissolution | With polar molecules only | With nonpolar molecules only |
| Bond type between nonmetals | Polar covalent bond | Nonpolar covalent bond |
3 Steps to Determine if a Molecule is Polar Or Nonpolar

1. Draw the Lewis Structure
The Lewis dot structure provides a simple model between the bonds in a molecule and the lone electron pairs. It’s essential for predicting molecular geometry, molecule polarity, and reactivity in a compound.
Begin drawing the Lewis dot structure of the molecule. You may need a periodic table for this.
2. Determine the Shape
Polar and nonpolar molecules differ in their geometric shapes.
Shape | Polarity | Compound Example |
Linear | Non-polar | C2H2 |
Tetrahedral | Non-polar | C2H4 |
Trigonal Planar | Non-polar | CH4 |
Bent/V-shaped | Polar | NH3 |
Trigonal Pyramidal | Polar | Water |
Nonpolar and polar molecules exhibit some degree of electronegativity difference between bonded atoms.[1]
Nonpolar bonds: The electronegativity values are equal or nearly equal. There’s no element with more negative or positive charge than another side. The electronegativity difference between bonded atoms is less than 0.4.
Polar bonds: The electronegativity difference is greater than 0.4 between bonded atoms. There is at least one side of the molecule with more negative or positive charge than another side.1.
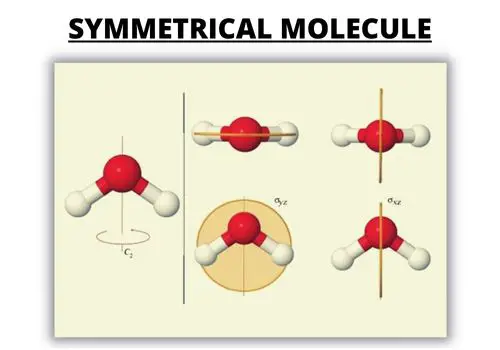
3. Dissect the Symmetric Molecules
Polar molecules have an unshared electron pair while the nonpolar molecule doesn’t. If you look at pictures of polar and nonpolar molecules, they vary in symmetry.
Examine the arrows in the Lewis dot structure.
Nonpolar covalent bond: The arrows are equal in length, and the arrangement is symmetrical. All the atoms attached to the middle atom are identical. They share all electron pairs.
Polar covalent bond: The arrows are of different lengths, and the arrangement is asymmetrical or uneven. The atoms attached to the atom aren’t all the same.
Some Examples:

Polar
Some examples of polar molecules include ethanol, methanol, water, hydrogen sulfide, hydrogen cyanide, sulfur dioxide, ammonia, ozone, sucrose, hydrofluoric acid, acetone, ether, acetic acid, difluoromethane, carbon monoxide, formaldehyde, methylamine, bromomethane, chloroform, hexane, acetonitrile, pyridine, glycerol, and butyl cellosolve.
Nonpolar
Some examples of nonpolar molecules include noble gases, carbon dioxide, methane, benzene, homo-nuclear diatomic molecules, oxygen, propane, butane, sulfate, carbon tetrachloride, ethylene, hydrocarbons (toluene, gasoline), sulfur hexafluoride, beryllium dichloride, acetylene, fats, turpentine, and alkanes.
Recommended Posts:
Are There Exceptions to the Rules?

The Lewis dot structure isn’t applicable with hydrocarbons and molecules consisting of two atoms of the same element. Lewis structure isn’t suitable if:
- Molecules have an odd number of electrons (e.g., NO).
- Molecules in one or more atoms have more than eight electrons (e.g., SF6).
- Molecules with less than eight electrons; an example is the BF3.
There are molecules with a polar bond, but the molecular geometry is symmetrical. As a result, they are nonpolar molecules by nature (examples: CO2, SO3).
There are molecules with a nonpolar bond but are polar molecules by nature (examples: NCl3, ozone, water).
Final Thoughts on Determining Polar and Nonpolar Molecules
For many students, understanding bond polarity can be a hard part of their high school chemistry subject. To determine if something has a nonpolar or polar bond, there are rules and steps to follow. However, there are always exceptions.
A molecule is polar if there’s a significant difference in the electronegativity charges between elements. The bonds don’t cancel each other out and are asymmetrical.
A nonpolar molecule has no separation of electric charges or difference in electronegativity. The bonds cancel each other out, are symmetrical, and there’s no lone electron pair.
This topic will be easier to grasp with consistent practice and a peer or teacher to help you out.
Thank you for reading this far! I hope that the information provided in this article will be helpful to you.
References:
- https://pubs.acs.org/doi/abs/10.1021/acsomega.0c00831

