Is Ammonia molecule polar? Many ask, considering the chemical formula comprises two nonpolar molecules – Hydrogen molecules and Nitrogen molecules.
The Lewis structure, electron geometry, and molecular geometry of this molecule made a considerable difference to the turn of events.
For better understanding, let’s find out the answer to the question – is NH3 polar or nonpolar?
Table of Contents
Polarity or Nonpolarity of NH3 (Ammonia)
NH3 or more commonly known as Ammonia has polar molecules. When the two atoms had chemical bonds, they are now asymmetrical molecules that have polar bonds.
NH3 or Ammonia is a colorless gas, a stable hydride formed of one atom, Nitrogen, and three hydrogen atoms. Ammonia has a pungent smell and a fertilizer content as a cleaning chemical and nitrogenous compounds.
Sources of this polar molecule are vegetable matter and nitrogenous animal waste products.
However, during rain, a measure of small quantities of ammonia and ammonium salts are found.
NH3 Molecular Structure
With the help of Lewis structure, we will understand electron geometry, polarity, and other properties of both polar molecules and non-polar molecules.
The bonding pair of electrons, or those that build bonds, create the shape of the NH3 molecules.
Before we get to the shape of Ammonia molecules, we have to find out the valence electrons that form dipole moments.
Nitrogen has five electrons in its outer shell, which are the valence electrons, while Hydrogen atoms only have one valence electron, but in this group, we have three with a central atom.
Therefore we multiply it by three as well, making our total valence atoms eight.
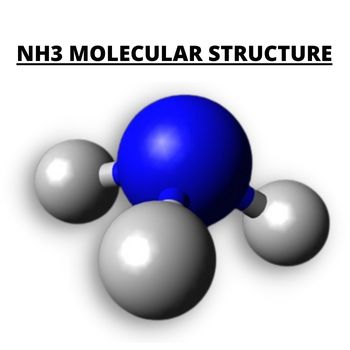
Since we now have the total valence electron to set the net dipole moment, the overall shape of NH3 is a Trigonal Pyramidal shape.
The Trigonal Pyramidal structure has a Nitrogen atom as a central atom with an asymmetrical charge distribution with three bonds and a lone pair.
Factors That Determine NH3 Polarity
Geometry
As discussed, the molecular geometry of Ammonia is tetrahedral shape. The nonpolar molecule is arranged symmetrically all over the Nitrogen base.
The two nonpolar molecules formed the molecular geometry of NH3 trigonal pyramidal.
Note that the distorted shape is because of the lone pair of electrons(1), which exerts repulsive forces on the bonding pairs. Although the bond angle should be 109.5 degrees for trigonal pyramidal molecular geometry, it went down to 107 degrees because of the lone pair on the nitrogen atom.
Electronegativity
The electronegativity difference is the first defining factor of a covalent molecule once they distribute unequal charges. The more electronegative the atoms on these covalent bonds are, the greater the polarity, just like what we have with nitrogen atoms.
Related Posts:
- Number of CS2 Double Bonds
- Number of Lone Pairs in Sulfur
- Number of Electron Lone Pairs on the S Atom
- Determining the Polarity of a Molecule
- Molecular Geometry vs Electron Pair Geometry
Dipole Moment
Mathematically speaking, the product of the charge over the atoms and the distance between them is the dipole moment of a molecule.
The dipole moment of N−Hbond will be from H to N. The net dipole moment of three N−H bonds will add up to 1.4 D. As we know that 1 D = 3.33564×10−34 C m.
In NH3, dipole moments are calculated around 1.46D with their asymmetrical shape of the polar molecule. These chemical compounds form polar bonds to connect their atoms and form asymmetrical molecules.
Read: Is SF4 Polar?
FAQS
Yes, we can say that NH3 is a polar covalent bond. Nitrogen forms a covalent bond with three atoms to form a molecule.
You can get the difference between the N-H bond and the NH3 compound since the two are polar, even in their gaseous state. In the bond N-H, the difference in electronegativity between N- and H- atoms is a negative charge.
And in NH3, the lone pair of electrons on the N-atom makes the form of Ammonia, triangular pyramid
Yes. Although NH3 shows dipole-dipole intermolecular forces, the highly electronegative atoms resulted in polarity. That is why NH3 is still a Hydrogen bond.
The atom bonded to them, and this polarity is categorized in its intermolecular force.
One thing to consider is that the amount of hydrogen bonding is limited since the nitrogen atom only has one lone pair.
So, Is NH3 Indeed Polar?
The answer to the question is a definite yes.
Partial charges are unevenly dispersed all around the molecule, with the negatively charged atoms at the top part of the molecule bond.
It is a polar molecule when the constituent atoms at one end of the molecule have a net positive charge. In contrast, a net negative charge is on the other end of the molecule, such as nitrogen and hydrogen atoms.
The bent form of the polar molecule caused by the repulsion of electron-electron caused a strong partial charge. Fact is, this is even a stronger polar net dipole moment. An additional thought – there are other hydrogen bond types, but among all, NH3 is the weakest. As a result, there is a large difference in electronegativity, making Ammonia molecule colorless gases at room temperature.
Again, NH3 is a polar molecule because it has three dipoles, and these dipoles do not cancel out each other, plus they have net dipole moments.
Thank you for reading this far! I hope that the information provided in this article will be helpful to you.
References:
- https://courses.lumenlearning.com/cheminter/chapter/electron-dot-diagrams/