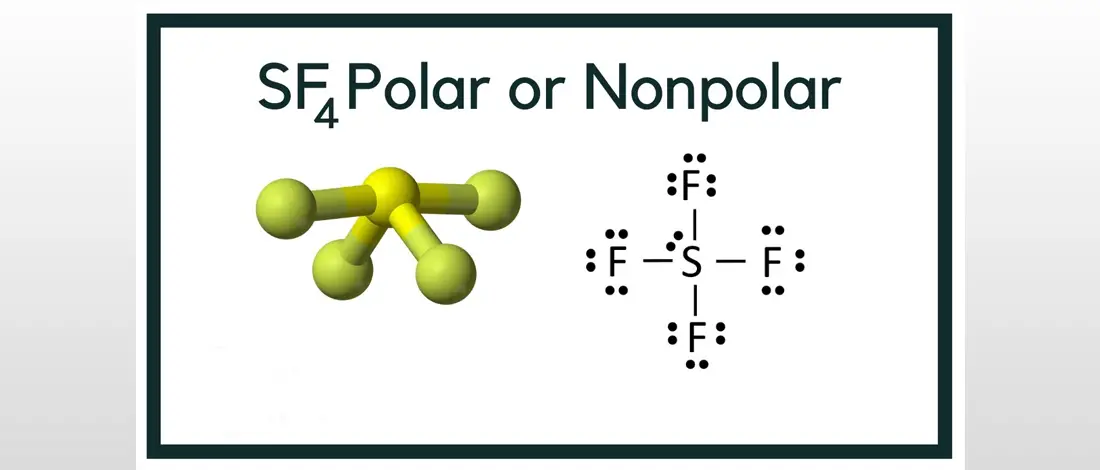
Sulfur tetrafluoride is a chemical compound composed of a central atom of Sulfur and 4 Fluorine atoms. The chemical formula for this compound is SF4.
Is SF4 polar or nonpolar? Let us all find out.
Table of Contents
Polarity or Nonpolarity of Sulfur Tetrafluoride
Sulfur tetrafluoride is a colorless gas with a recognizable Sulfur odor [1]. The melting point of these molecules is −121.0 °C. The boiling point of these molecules is −38 °C. SF4 is helpful when preparing for the Organofluorine Compounds.
The Sulfur tetrafluoride is a polar molecule because Fluorine is more electronegative than Sulfur. With this, the distribution of the charge is not equal, making the SF4 polar molecules.
The molecular geometry of SF4 is in a seesaw molecular shape that can be seen when you draw the Lewis Structure. Since four Fluorine atoms are more electronegative than Sulfur atoms, there will be an uneven distribution charge. If the lone pair is odd, it is polar; while a lone pair is even, it is nonpolar. The molecule of the Sulfur tetrafluoride is polar because there is one lone pair.
SF4 Molecular Structure & Hybridization
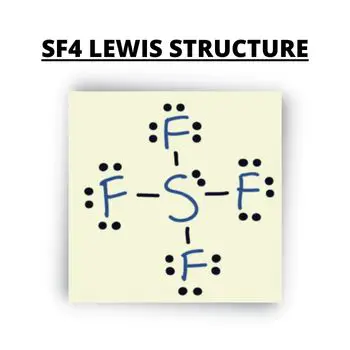
The molecular structure is the atoms, ions, or groups in a molecule that is relative to one another. It also includes the number and location of chemical bonds. The molecule of SF4 consists of 34 valence electrons. The six valence electrons will come from the central atom Sulfur, and the 28 electrons will come from 4 Fluorine atoms with 7 electrons each.
It is essential to understand the Lewis structure and the number of valence electrons present to identify the SF4 Hybridization. The central Sulfur atom will form a covalent bond with each Fluorine atom, wherein it uses eight valence electrons. The four Fluorine atoms with three lone pairs in octet will utilize the 24 valence electrons.
In addition to this, the two electrons in the Sulfur atom will remain as one lone pair. The center Sulfur atom consists of 5 valence electrons that will form SP3d due to hybridized orbitals. There will be four single bonds in the Sulfur atom and one lone pair. With this, the number of regions of electron density is 5.
Elements That Determine SF4 Polarity

Geometrical Shape
The first element that can identify if the Sulfur Tetrafluoride is polar is by its molecular geometry. To identify this, use the Lewis Structure. The seesaw shape of this molecule is due to the lone pairs of electrons. The molecular geometry of SF4 is seesaw with an asymmetric distribution of electrons around the central atom.
The geometry shape of SF4 is Trigonal Bipyramidal. The geometrical shape of the molecule is asymmetric because they have unequal charge distribution. With this, the Sulfur Tetrafluoride is a polar molecule.
Electronegativity
Electronegativity is the measurement of the atom’s ability to attract shared electrons. It increases as you move from the left to right of the periodic table and decreases when you move down.
When it comes to the electronegativity of the Sulfur tetrafluoride, F is around 3.98, and S is at 2.58. While the Fluoride has a partial negative charge from the negative pole, the Sulfur atom has a partial positive pole, creating the bonds with a polar charge.
Related Posts:
- Is NH3 Polar or Not?
- Is CH2Cl Polar or Not?
- What’s the Molecular Geometry of BrF3?
- How to Know If A Molecule is Polar or Nonpolar?
- What’s the Lewis Structure of I3?
Dipole Moment
The dipole moment happens when there is a charge distribution. It occurs between two atoms in a covalent bond. Notice that when there are differences in electronegativity, the dipole moment arises. The larger the moment means there are more significant differences in the electronegativity of the molecules.
To calculate the moment of the SF4, multiply the charge on atoms and the distance between positive and negative charges. The SF4 dipole moment is around 0.632 D. Since the dipole is not equal to zero, SF4 is polar.
FAQS
The type of bond for SF4 is a covalent bond. The Sulfur tetrafluoride consists of 1 central atom, Sulfur, and 4 Fluorine atoms with 34 valence electrons. It forms four covalent bonds and one lone pair of electrons, as seen when using Lewis Structure. The Fluorine atom equatorial is 102 bond angle, and the Fluorine atom axial is 173 bond angle.
Yes, SF4 is a dipole. In the Lewis Structure, the unsymmetrical seesaw structure of SF4 leads to an overall dipole. Due to the two nonbonding pairs of electrons, SF4 is a dipole. In addition to this, the dipole moment of this chemical compound is not equal to zero.
Determining the Polarity or Non-polarity of SF4
The SF4 is polar, and it was determined using different elements. Drawing the Lewis Structure led us to the molecular geometry of the compound. Who would have thought that these molecules’ shape is like trigonal bipyramidal? Related questions regarding the polar molecule SF4 also gave us a lot of learning today.
Thank you for reading this far! I hope that the information provided in this article will be helpful to you.
References:
- https://pubchem.ncbi.nlm.nih.gov/compound/Sulfur-tetrafluoride#section=3D-Conformer