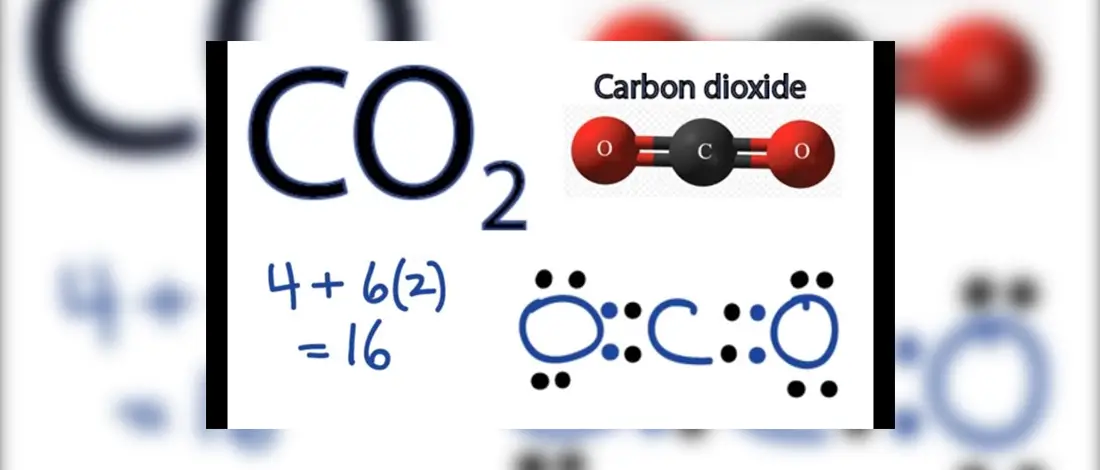When we were younger, our understanding of CO2 was usually associated with breathing oxygen and releasing CO2. That is true, but Carbon Dioxide is not just the colorless gas that smells.
It is also a good starting point in learning how to fully understand the CO2 Lewis Structure and Molecular Geometry.
Table of Contents
Understanding the CO2 Lewis Structure
You need to understand the Lewis Structure or Lewis Dot Structure of a molecule to find out how the atoms form bonds and produce the shape of the molecule. For CO2, the Best Lewis Structure is to find the charged atoms.
The number of valence electrons of a carbon atom is four which forms four bonds. The CO2 Lewis Structure is where the central carbon atom is the central atom, the least electronegative atom with O surrounded by four dots.
The total valence electrons, including valence shells, are eight electrons pairs with no lone pair.
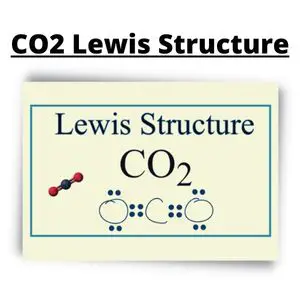
Closer Look at the Structure
Hybridization
CO2 has SP Hybridization. Carbon atoms have a different electronic configuration from Oxygen atoms which affects CO2’s SP hybridization. This changes when the electrons are in their excited state, where it becomes 1s2 2s1 2p3 where one electron goes to each p-orbital.
The hybridization between 2s orbitals and one of the p-orbitals will form 2sp orbitals, which resulted in sigma bonds formation. This will form the sigma bond because of the two p-orbitals that are overlapping.
Molecular Geometry
CO2 has an AX2 molecule based on the VSEPR theory making the molecular geometry of CO2 linear. The linear molecular geometry has a symmetrical structure with bond angles of 180 degrees once it takes its linear shape. The valence shell electron pairs also cause repulsive forces hence the shape of the molecule.
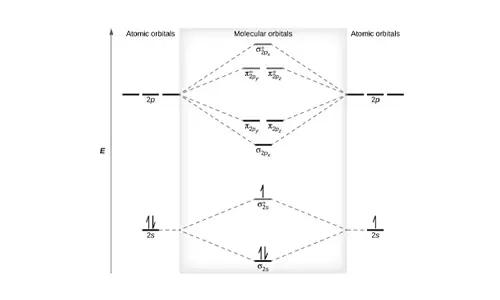
Molecular Orbital (MO) Diagram
In a Molecular Orbital Diagram, the 2s orbital of oxygen is nonbonding because of the high energy difference between carbon and oxygen atoms.
Based on the rules of the Lewis Structure, all 16 electrons are filled upon bond formation, but the nonbonding orbitals remain vacant, as in the case of CO2.
Bond Angle
CO 2 has double bonds, coming from Carbon and Oxygen. Two oxygen atoms are at the terminal ends of the bond, while the central carbon atom has an angle bond length of 180°. There are eighth electron pairs in this bond and no lone pair as shown in the Lewis Structures.
Polarity
The CO2 molecular geometry is linear in shape, which means that the double bond has the same influence on the charge, making CO 2 a nonpolar molecule. There is no dipole moment generated between the two oxygen atoms making this not a polar molecule. Carbon Dioxide(1) has two double bonds, which contribute to its linear geometry.
Also Read:
Preparation Process
There are various processes in preparing CO 2.
1. This is where calcium carbonate reacts with hydrochloric acid, which is a very common method used in laboratories.
2HCl + CaCO3 ——–> CO2 + CaCl2 + H2O
2. Mixture of methane and oxygen
CH4 + 2O2 ——-> CO2 + 2H2O
3. This process uses combustion, where carbon-based fuels are produced through thermal decomposition. Calcium Carbonate goes through the heating process and forms quicklime which is commonly used in industries.
CaCO3 ——-> CaO + CO2
4. With the use of Carbonic Acid through decomposition.
H2CO3 ——–> CO2 + H2O
Molar Mass
Carbon Dioxide also known as CO2 has a molar mass of 44.009 g/mol with a density of 1562 Kg/m3.
Acid or Base
CO2 or carbon dioxide is considered as acid or can even be called Lewis Acid.
The resonance structure accepts lone pairs of electrons, but the three lone pairs of electrons are in the oxygen molecule.
There are no lone pairs of electrons in CO 2. Although, when dissolved in water, CO 2 takes the form of carbonic acid using this formula.
CO2(g)+H2O(l)⇌H2CO3(aq)

6 Steps on How to Draw CO2’s Lewis Structure
- Calculate the total valence electrons found in a molecule.
- Carbon Valence Electron=4
- Oxygen Valence electrons: 6*2 = 12
- Total number of valence electrons = 16
- Find the central atom, which is usually the one with the highest bonding sites, is the Carbon atom. Draw four dots around it.
- The remaining electrons, beginning with the electronegative atoms and then the electropositive, should complete the octet rule of the atoms.
- Since an oxygen atom needs extra electrons, the carbon atoms share electrons with the oxygen atom and form a double bond. This central atom will now have a double bond with the oxygen atom.
- Two parallel lines on both these atoms are an indication of the double bonds.
- The last part is to make sure that the atom’s lowest possible formal charge is achieved. Below is the formula for calculating the formal charges. FC = VE – [LPE – ½(BE)]
FAQS
CO2 is a linear molecule as there are no lone pairs since there are four electrons bonded. Two double bonds are trying to get as far apart as they can. These are atoms that have a formal charge, but not all the atoms have an octet configuration.
The VSEPR shape of CO2 is Tetrahedral since multiple bonds, either a double or triple bond, take one electron from the final total. The same atoms are recognized by VSEPR, which can be seen in the Lewis Structure.
CO 2 has two electron domains that consist of bonding pairs. One carbon atom is the center atom, while the Oxygen atom has two lone pairs each. The valence electrons on each carbon atom are four which are double bonding with the oxygen atoms.
Key Takeaways
Carbon Dioxide or CO 2 is not only a contributing factor to greenhouse gas in Earth’s atmosphere because there are many uses for this trace gas. Understanding the individual atoms and CO2 Lewis Structure will give you a better grasp of its purpose.
The CO2 Lewis Structure can be confusing initially, but if you can find out the valence electrons of both carbon atom and the oxygen atoms, the Lewis Structure, its electron density, and molar mass will no longer be a confusing aspect for you.
Thank you for reading this far! I hope that the information provided in this article will be helpful to you.
Reference:
- https://climate.nasa.gov/vital-signs/carbon-dioxide/