BF3 is an inorganic compound with an interesting behavior in every state of matter. It can be in a liquid state, highly soluble, but it can also be toxic when in a gaseous state as it creates white fumes in moist air.
Let us learn more about BF3, its molecular geometry, hybridization, bond angle, and how to draw the best Lewis Structure for it.
Table of Contents
BF3 Lewis Structure: What You Need To Know
We need to know how to draw the Lewis Structure of Boron Trifluoride to find out the arrangement of the electrons in both molecules present.
will start with the periodic table and get the atomic number of Boron and Fluorine to calculate how many valence electrons are present in the chemical compound.
The computation will also present how these electrons bond (single bond or not) and the lone pairs if there are any created.
You will see how it violates the Octet Rule while drawing Lewis Structure.
Understanding Its Properties & Structure

Hybridization
Hybridization in pairs of electrons happens when you combine atomic orbitals and new hybrid orbitals. It will be explaining the bonding properties of the nucleus and its molecular geometry in the Lewis Structure.
BF3 has SP2 hybridization since Boron (1) requires one π (pi) bond to double bond, but it only formed three σ bonds per atom. Orbitals S- and P- in the outer shell of Boron mix to create another three SP2 equivalent hybrid orbitals.
Molecular Geometry
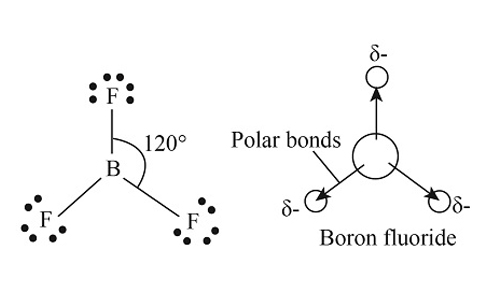
BF3 has fluorine atoms at the top of the equilateral triangle formed with Boron atoms. Thus, its molecular geometry is Trigonal Planar. You will see three atoms that single bond with the central atom. These are three BF bonds at 120o and in the same plane. You will not see three lone pairs in BF3 since all three electrons bonded with the Boron atom.
Also Read: BrF3 Molecular Geometry
Bond Angle
The polarity or non-polarity of a molecule depends highly on its structure and shape.
The bond angles of the electrons determine these. BF3 has a bond angle of 120°, with all atoms aligned in one plane.
A fluorine atom is positioned at 120°, which is bonded with the center atom Boron in the Lewis Structure.
Based on the chemistry and VSEPR model, this is the best Lewis Structure formed with the single attachments to the central molecule.
Polarity
You can determine the polarity of the molecule with its electronegativity. In the case of Boron and Fluorine, their 0.5 electronegativities are categorized as nonpolar. If we look at the symmetrical structure and the geometry of BF3, it will result in a zero molecular dipole moment, which makes it nonpolar. Regardless of the Lewis Structures or Lewis base pairs of electrons, BF3 is nonpolar.
Related Posts:
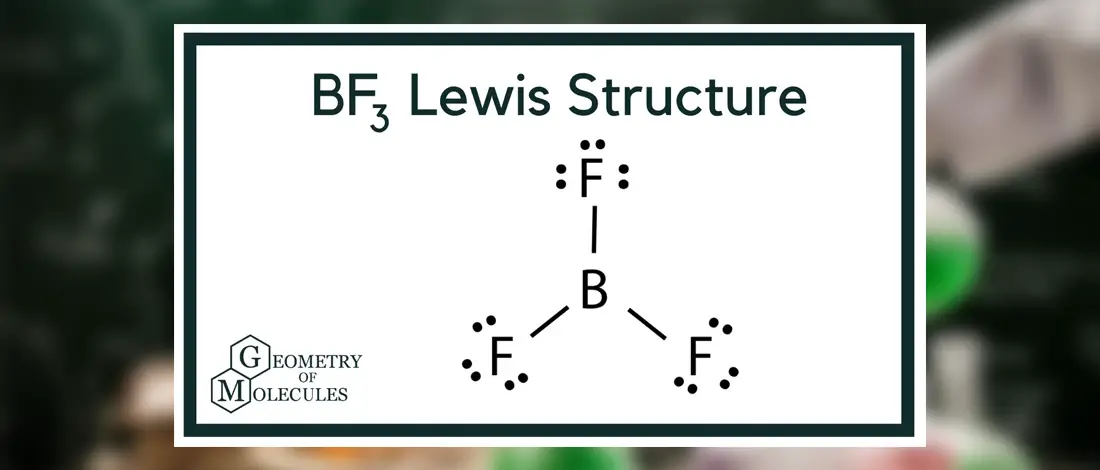
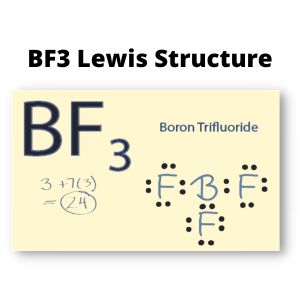
Drawing The Lewis Structure of BF3
In BF3, Boron is the least electronegative atom and will be used as the central atom. The Lewis Dot Structure will show you one Boron atom with three electrons in its last shell and three Fluorine atoms with seven electrons in its last shell. The computation will end with 24 total valence electrons, forming three B F bonds.
Boron – 2, 3
Fluorine – 2, 7
3 + 7 (3) = 24
According to the Octet, Boron requires six valence electrons around its outermost shell, while Fluorine (2) needs 8.
6 + 8 (3) = 30
Find the difference between the total valence electrons and required electrons.
30 – 24 = 6
This time, get the number of lone pairs from the two atoms by calculating the difference between bonding pairs electrons and the number of valence electrons.
24 – 6 = 18
Once the number of valence electrons is computed, you place B as the central atom with three single bonds with F atoms. This will form your Lewis Structure.
FAQS
No, Fluorine will not be the center atom of BF3 because it is more electromagnetic than Boron. Also, Fluorine atoms never form double bonds but only assign all atoms to zero formal charges.
BF3 does not have a lone pair because there are only six valence electrons. Plus, it disobeys the octet rule since it has the second-period covalent molecules. You will find three bonded groups, meaning it has trigonal geometry, therefore, no lone pairs.
There are six valence electrons around Boron in BF3 based on the drawing of the Lewis structure. It means that Boron’s octal is not complete, which is a very rare instance. Therefore, BR3 is considered a Lewis acid.
Key Takeaways
BF3 or Boron Trifluoride is an inorganic compound that lacks Carbon. This created no lone pairs between the Boron atom and Fluorine. It can sometimes be confusing whether BF3 is polar or nonpolar because the three Fluorine atoms have much higher electronegativity than the Boron atom.
BF3 Lewis Structure showed the total number of B F bonds and the total number of electrons present. It also showed the center atom formed single bonds in a trigonal planar shape. Therefore, each molecule, computations, and process that produced this Lewis Structure have followed every rule set in chemistry.
Thank you for reading this far! I hope that the information provided in this article will be helpful to you.
References:
- https://www.rsc.org/periodic-table/element/5/boron
- https://www.rsc.org/periodic-table/element/9/fluorine