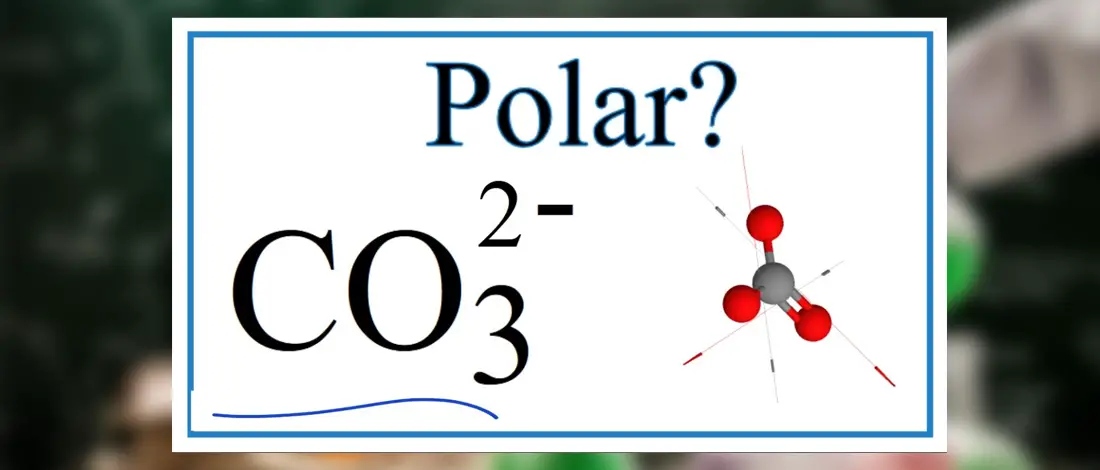
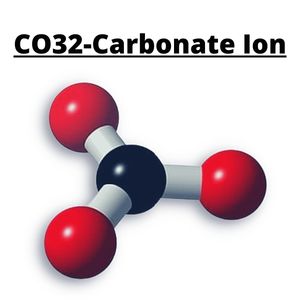
CO32- or Carbonate is a polyatomic ion. It is made of three Oxygen atoms bonded with the Central Atom, which is Carbon. These bonded atoms are polar in nature, but CO32 is nonpolar.
How did this happen?
Let us dissect the facts and learn more about CO32-, its electronegativity, geometric shapes and charges, and its Lewis Structure to determine why we say such.
Table of Contents
CO32: Is It Polar Or Nonpolar?
CO32- or Carbonate Ion is a Carbon Oxoanion and a base conjugate of hydrogen carbonate.
When Carbonic Acid (CO2) dissolves in water, inorganic Carbonate is formed. However, CO32- belongs to both inorganic and organic classes.
CO32- is made of the Central atom, Carbon, and Oxygen atoms which have formed polar bonds.
Based on the Pauling scale, the charge difference between the atoms that have bonded is 0.89.
However, it has an asymmetric shape that causes the charges to be distributed evenly. Thus it is nonpolar in nature.
Closer Look at its Properties
CO32- Bond Angle/Nature
To determine whether CO32- is polar or nonpolar, you need to check the periodic table to determine which bonds are present. Based on the table, the charges of Oxygen and Carbon have a difference of 0.89. If we base it on the ruling of the Pauling Scale, CO32- is polar because of the bond between the Oxygen atoms and Carbon atoms.
CO32- Molecular Symmetry & Shape
Based on VSEPR theory, as the Oxygen atoms repel each other, it spreads away from each other in a consistent manner. The Central Carbon Atom is bonded with three Oxygen atoms in CO32, resulting in equal bond angles of 120°. This makes the structure of CO32- symmetrical in nature and has Trigonal Planar geometry or shape.
CO32- Electronegativity
You can find two elements in Carbonate (1), Carbon and Oxygen. Oxygen has an electronegativity value of 3.44, while Carbon only has 2.55. Since we follow the common procedure in choosing the central atom, the one with the least electronegativity will be considered the Central Atom. Therefore, the Central Atom of Carbonate Ion is Carbon.
CO32- Negative 2 Charge
The nature of CO32- is symmetrical, which means its polar bonds are evenly distributed. This also means that it has zero net dipole moment because of induced charges but has canceled out. Basing it on the dipoles in opposite directions would show that CO32- contains a negative two charge.
Related Posts:
- Is NH3 Polar or Nonpolar?
- Is CH2Cl2 Polar or Nonpolar?
- Is BCL3 Polar or Nonpolar?
- Is CH3OH Polar or Nonpolar?
CO32- Net Dipole Moment
Based on processes, dipole moments refers to the electrons distributed among the bonded atoms. Dipole moment exists if there is a difference between the electrons with polar and nonpolar bonds, which concludes that polar molecules have net di[pole moment. Carbonate, Carbon electrons, and Oxygen electrons were distributed evenly, making it planar. Therefore, it has no net dipole moment.
CO32- Molecular Geometry
With Carbonate, we use the AXn notation.
A= Central Atom
X= number of atoms around the Central Atom
n= number of bonds that are attached to the Central Atom
Ex= the number of lone pairs of the central atom
If we compute for the Molecular Geometry of Carbonate
A= Carbon atom,
X= Oxygen atom,
n= 3,
Ex= 0.
We will reach the notation AX3.
Using the VSEPR chart, AX3 notation in CO32- shows a Trigonal Planar with a bond angle of approximately 120°.
CO32- Hybridization
Atomic Orbital has a special function in an atom’s quantum mechanics and theory. When chemical bonding occurs, AOs combine and create bonds inside the molecule and form hybridized orbitals.
In the Lewis Structure of CO32, a single O atom bond has a negative charge while the double-bonded ones have no charge value. You will see three sigmas and one pi around the central C atom.
The formula below shows how to compute the Hybridization value.
V = 4, M = 0, C = 0, A = 2.
H = 0.5 ( 4 + 0 – 0 + 2 ) = 3.
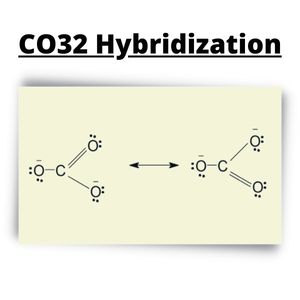
CO32- Lewis Structure
To develop the Lewis Structure of CO32-, we follow the steps and draw an appropriate CO32- diagram.
Step 1: Find out the total Valence Electron present (based on the periodic table)
= 4 + 6*3 + 2
= 24.
Step 2: Find out the molecule’s Central atom.
Since Carbon is the least electronegative among the atoms found in Carbonate Ion, it becomes the central atom.
Step 3: Drawing the Molecule’s Skeletal Diagram
Using dots as valence electrons around the atomic symbols, you can place the corresponding number of valence electrons around three Oxygen atoms surrounding the Central Atom, Carbon.
Step 4: The Formed Bond formation
The total Valence Electron is 24, while there are two shared electrons between Carbon and the three Oxygen atoms, which have single bonds. Therefore the Octet Rule has been fulfilled with Oxygen Atoms but not with Carbon that only has six electrons.
Step 5: Formal Charge
The formula for formal charge:
Carbon= 4 – 0.5*8 – 0 = 4 – 4 = 0.
Each O Atom Formal Charge= 6 – 0.5*2 – 6 = 6 – 1 – 6 = -1.
Each O Atom with double bond with carbon = 6 – 0.5*4 – 4 = 6 – 2 – 4 = 0.
FAQS
Carbonate ion is a covalent compound that has a negative two net charge. This is a negative ion or anion.
The bond of CO32- is polar in nature. Based on the Pauling scale, the charge difference between the atoms of Oxygen and Carbon is 0.89. Therefore, the bond is polar.
There are three lone pairs in CO32- or Carbonate. It comes from the Oxygen atom since the central atom, Carbon, does not contain lone pairs.
CO32- Polarity Result
Carbonate (CO32-) is an organic and inorganic class, making it a versatile yet complex compound. What makes CO32- more interesting is how these bonds. As CO32- ion, these are polar in nature because all three Oxygen bonds are polar. If this is the only basis, it will make Carbonate polar.
However, these bonds have negative charges and constituents that are also evenly distributed because of their symmetric shape, causing zero net dipole moment. Because of that, by nature, CO32- ion is nonpolar.
Thank you for reading this far! I hope that the information provided in this article will be helpful to you.
Reference:
- https://pubchem.ncbi.nlm.nih.gov/compound/Carbonate#section=2D-Structure