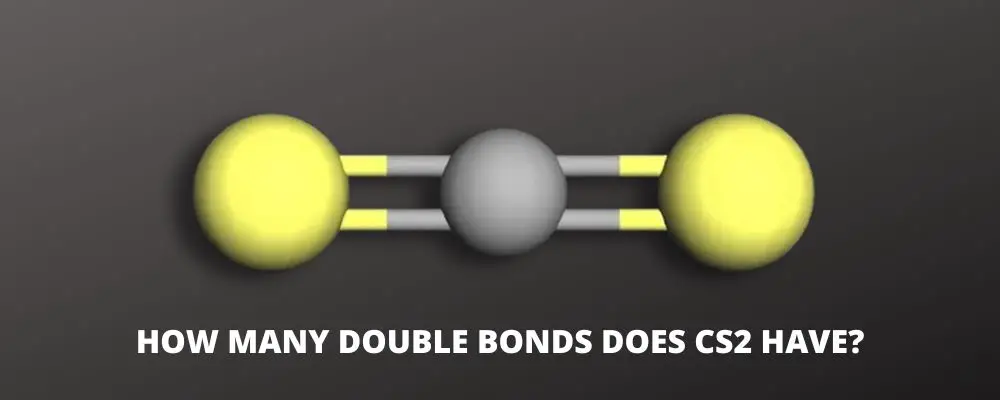
Carbon disulfide (CS2) is an extremely volatile, flammable, odorous, and colorless compound. But, how many double bonds does CS2 have?
For a more concise discussion on CS2 Lewis structure, bond type, and other molecular properties, continue reading.
Table of Contents
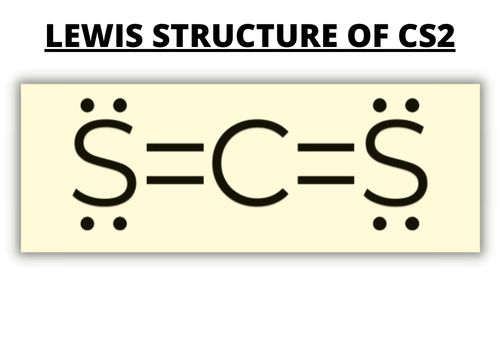
Understanding the CS2 Lewis Structure
The Lewis structure is a very simplified illustration of the valence shell of electrons in a molecule using dots and lines. It follows the octet rule concept.
In the periodic table, only the s and p elements are involved in the octet rule and Lewis structure.
Looking at the CS2 Lewis structure, CS2 is composed of one carbon atom and two sulfur atoms. Carbon atoms have the least electronegative charge; therefore, the carbon is in the center bonding with the two sulfur atoms forming the bond angle.
The Lewis structure of a carbon disulphide molecule is key to understanding its geometry, type of bond, polarity, and hybridization.
CS2 Bond Formation and Its Type
Chemical bonds are classified into three in terms of their interactions:
- Covalent: Sharing of valence electrons between nonmetals.
- Ionic: Transfer of valence electrons between a metal and a nonmetal.
- Metallic: Chemical bonding between metals.
There are three types of bonds based on the number of pairs of electrons:
- Single: Two atoms share one electron pair.
- Double: Two atoms share two electron pairs.
- Triple: Two atoms share three electron pairs.
The Lewis structure can help you determine at face value the bond formation between atoms.
The CS2 molecule has two nonmetals (i.e., carbon and sulfur); therefore, it’s a covalent bond.
The sulfur atoms form double bonds with the central atom.
The molecule has a total of 16 valence electrons; four valence electrons from the carbon atom and six valence electrons from each sulfur atom. The remaining eight electrons are the lone pairs. The valence electrons are in 2p and 2s orbitals of the central atom.
Other Factors to Consider

Polarity
Polarity refers to the distribution of electrical charges across the molecule or atoms of a molecule.
Polarity is determined by the electronegativity values of two atoms involved in a bond.
A polar molecule results from unequal sharing of electrons, whereas a nonpolar molecule results from the equal sharing of electrons between two atoms.
CS2 molecule is nonpolar even though it has lone pairs of unshared electrons, and its carbon-sulfur bond differs in electronegativity. The charge gets canceled due to its linear symmetry. You’ll find an in-depth discussion of polar vs nonpolar molecules here.
Molecular Geometry
VSEPR theory is essential to determining the molecular geometry of CS2 molecules.
It posits that each atom in a molecule will have a geometry or structural arrangement that reduces the repulsion between electrons in the valance shell of that atom.
CS2 molecule has a linear shape with bond angles of 180o. The carbon atom is between the sulfur atoms.
Hybridization
Hybridization is the fusion of atomic orbitals to create newly hybridized orbitals, which can influence bonding properties and geometry.
The CS2 molecule has “sp” hybridization.
From a theoretical standpoint, “sp” hybridization is formed if an “s” orbital overlaps with a “p” orbital. If the central atom has two valence electron density regions around it, it will produce sp hybridization.
You can use formulas to determine the hybridization of CS2.
Here’s the formula:
Hybridization value= 0.5(V+M-C+A)
- V: number of valence electrons in the central atom
- M: number of valence electrons
- C: charge of the cations
- A: charge of the anions
Therefore, H=2 here. It means that the hybridization type is sp.
Calculate the steric number. Here’s the formula:
Number of sigma bonds + number of lone pairs (of the central atom)= Steric number
2+0 (no lone pairs in the carbon atom) = 2
This means that there are two pi bonds formed or two hybrid orbitals- the sp hybridization of CS2.
Related Posts:
FAQS
The CS2 molecule is a nonpolar covalent bond with two double bonds. The valence electrons that form this type of bond are in 2s and 2p orbitals of the carbon atom.
Two atoms share one pair of electrons in a single bond only, whereas, in a double bond, two atoms share two pairs of valence electrons between each other.
Determining CS2 Double Bonds
Carbon disulfide is often used in soil disinfectants and can cause many health hazards, including death when induced in large amounts. [1] Not having basic chemistry knowledge can be a problem for any student. Understanding molecules or compounds requires some explanation about certain properties such as CS2 Lewis structure, bonding structure, polarity, molecular geometry, and hybridization.
We discussed the chemical properties of each carbon disulfide molecule. It has 16 valence electrons, has unshared lone pairs, and forms two double bonds. It’s a nonpolar covalent bond, possesses sp orbitals, and has a linear shape.
Thank you for reading this far! I hope that the information provided in this article will be helpful to you.
References:
- https://pubs.rsc.org/en/content/articlehtml/2017/cs/c6cs00585c