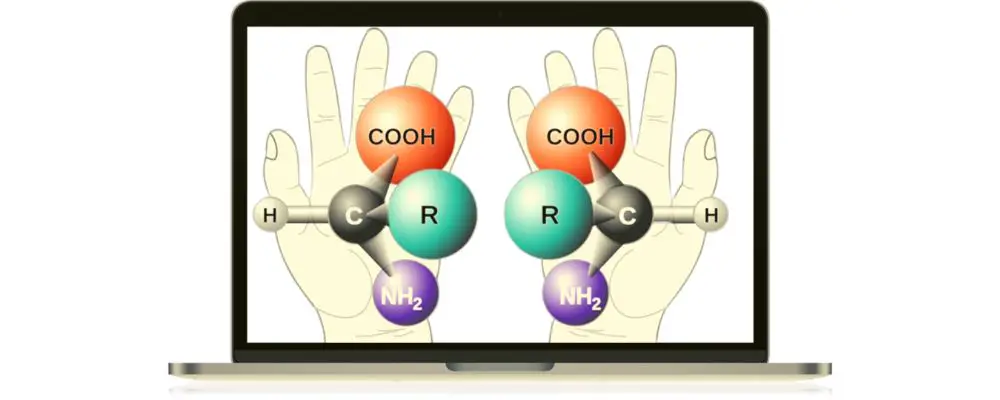
In organic chemistry, a molecule possesses various properties, and one of them is chirality. It pertains to a carbon atom with four different ligands or substituents. Knowing this concept is fundamental to understanding isomers.
Today, we will show you the steps on how to find chiral centers.
Table of Contents
2 Steps to Follow When Looking for Chiral Centers in Molecules
1. Eliminate atoms that cannot be chiral centers.
A chiral center is a stereocenter with an atom (usually carbons) attached to four nonidentical substituents. To spot potential chiral centers in a molecule, look for carbons connected to four different groups.
Exclude the atoms that aren’t considered chiral centers. These include all straight-chain alkyl group carbons (CH 3 or CH 2 units) because they have two or more identical atoms (i.e., the hydrogens) connected to the carbons. Oxygen, halogens, and carbons on double or triple bonds can’t be chiral centers as they can’t have bonds to four different groups.
Any molecule with chiral centers are mirror images of each other, but they are non-superimposable.
2. List out the groups or substituents.
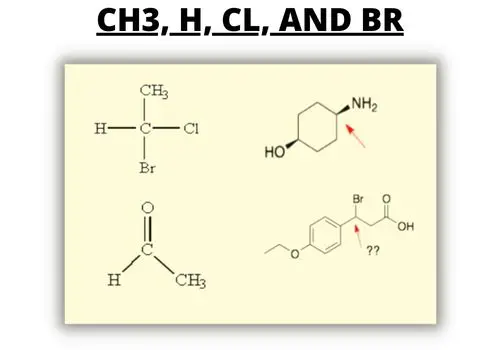
List out the groups attached to the carbon atom. Look at the following examples.
The four groups attached to the carbon are CH3, H, Cl, and Br. They are all different groups. Therefore, this is a chiral center.
The groups attached to this carbon atom are O, CH3, and H. There are only three groups. Therefore, it is not a chiral center.
The four groups attached to this carbon atom are NH2, H, CH2CH2CHOH, and CH2CH2CHOH.
The last two groups are the same. Therefore, this is not a chiral center.
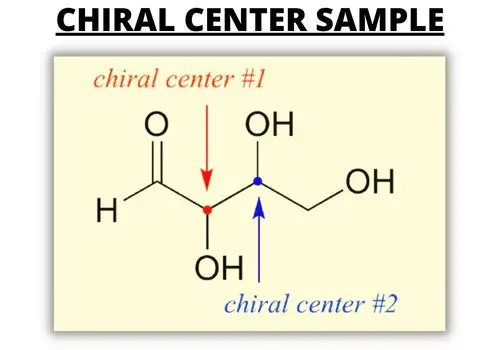
There seem to have two chiral centers or carbon atoms in this example, but the other carbon is attached to only three groups; therefore it is not chiral. For the second carbon, there are the four groups attached to the carbon atom: H, CH2, C, and Br. These are all different. Therefore, this is a chiral center.
For a compound with more than one carbon atom, consider the orientation of the chiral molecules. They have the same molecular formula and property but differ in orientation. Lactic acid has two chiral centers that are identical but have no symmetry plane and aren’t superimposable.
What are Chiral Centers?
In Chemistry, chiral centers are tetrahedral atoms bonded to four different substituents. They are usually carbons. Other types of atoms that can be chiral are tertiary nitrogens, quaternary nitrogens, hypervalent phosphorus, and hypervalent sulfur. However, these chiral atoms are rare.
A molecule or compound may have two chiral centers or more. Compounds with more than one chiral center are chiral but with the exception of Meso compounds.
Chiral centers are considered enantiomers, which are non-superimposable types of stereoisomers. They have the same molecular formula and connectivity but differ in orientation. They are mirror images, but they cannot be put on top of each other and become the same molecule. Just like the right and the left hands, they are identical, but they cannot be superimposed.
Related Posts:
Why are Chiral Centers Important?
Many of our biological molecules, including our DNA, amino acids, neurotransmitters, and glucose, are chiral molecules.
In making therapeutic drugs, the chiral center is crucial.[1] Different isomers in compounds cause different effects on the body.
One isomer of a chiral drug could treat a particular disease while others may be inactive or highly toxic.
Take, for example, the drug thalidomide. It used to be prescribed for pregnant women with morning sickness.
One enantiomer is effective at alleviating symptoms, but the other enantiomer can cause serious birth defects.
FAQS
A molecule is chiral if four different atoms are attached to a tetrahedral atom (usually carbons). A molecule may have two chiral centers or more. One example is lactic acid which has two chiral centers. The mirror image isn’t superimposable with the other image.
In organic chemistry, the feature that determines chirality is an asymmetric carbon atom in a molecule or compound. The mirror images or enantiomers of a chiral molecule cannot be superimposed with each other.
Determining Chiral Centers
Organic chemistry is a challenging course, but the concepts are highly relevant to our day-to-day lives. Some compounds have the same chemical formula and physical property but differ in structures and orientation in space (i.e., isomers).
We have shown you the steps in identifying stereocenters in a compound. Identify the carbon atom with four different ligands, and then examine the orientation and plane of symmetry.
You might have questions about the need to grasp these concepts. One good answer to that is helping us demystify why certain chemicals in foods or drugs can be both beneficial and toxic to our physical bodies.
Thank you for reading this far! I hope that the information provided in this article will be helpful to you.
References:
- https://europepmc.org/article/med/28356054