SF4 is known as the compound Sulfur Tetrafluoride – a colorless corrosive gas. SF4 is utilized in the synthesis of the organofluorine compound.
In molecular geometry and hybridization, how many lone pairs of electrons are on the s atom in SF4? Let’s find out!
Table of Contents
Determining the Lone Pairs of Electrons on the SF4 S Atom
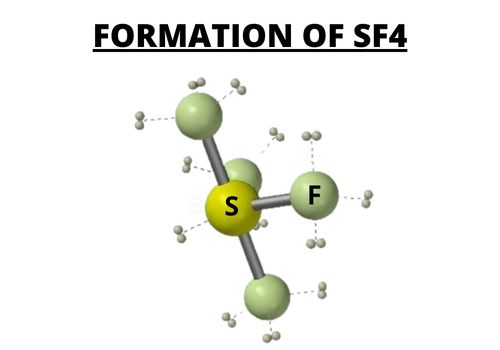
In the formation of SF4, the sulfur atom compound produces bonds with the fluorine where there are eight valence electrons.
In SF4, the four fluorine atoms have three pairs of electrons in the octet, which uses twenty-four valence electrons.
There are a total of thirty-four valence electrons. These valence electrons are for covalent bonding.
Two electrons are lone pair in SF4. You can determine the SF4 hybridization of sulfur compounds by considering the regions of the electron density.
When there is bonding, there is a formation of four single bonds in four single bonds in SF4, and it only carries one lone pair.
Sulfur is in the central atom. The central atom and the sulfur compound have a pair of electrons.
Factors to Consider

Molecular Geometry
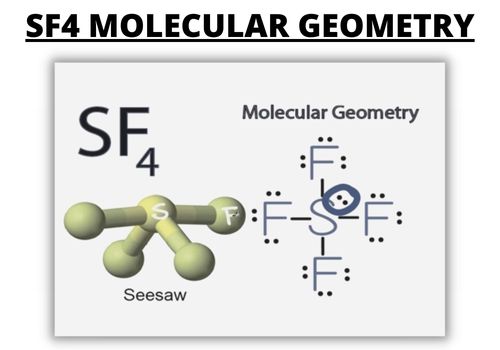
In SF4 molecular geometry, the molecular formula helps you to know the precise number and the type of atoms present in the specified compound.
There is a sulfur atom and four fluorine atoms in its compound, making it the same as the formula of the AX4E in SF4 molecular geometry.
The molecules having the formula of AX4E have the trigonal bipyramidal molecular geometry positions. Two fluorine atoms forming bonds with the sulfur atom are in the equatorial position. Meanwhile, the two are in the axial position in molecular geometry opposing the fluorine atoms.
When the central atom carries one or more pairs of electrons, the geometries of molecules change.
Hybridization
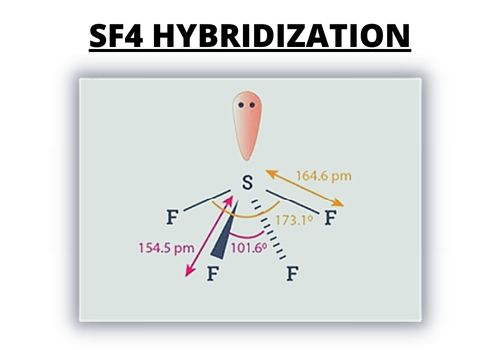
To find out the SF4 molecule’s hybridization, you have to consider the regions of the electron density of the central atom.
For hybridization, the Sulfur compound has four bonding pairs of electrons. It also has one lone pair. The sulfur atom utilizes five hybridized orbital, a 3s orbital, three 3p orbitals, and a 3D orbital.
The following arrangement of the electrons around them and the hybridized orbitals leads up to the sp3d hybridization. You can utilize the steric number as well for hybridization.
5 is the steric number for the sulfur atom.
Lewis Structure
Lewis Structure is a pictorial representation of bonds and the number of valence electrons in the molecule.
The number of valence electrons participating in forming bonds is known as the bonding pairs of electrons in the Lewis Structure.
Meanwhile, the electrons that do not participate are known as nonbonding pairs of electrons in the Lewis Structure.
In the Lewis structure of SF4, you need the total number of valence electrons in the molecule. There is a sulfur atom in the compound and four fluorine atoms.
To find out the total valence electrons of the compound, you need to know the valence electrons of the atoms individually in the Lewis Structure.
Related Posts:
Bond Angles & Shape
Central sulfur atoms have four bond formations with the nearby fluorine atoms.
It has one lone pair of electrons. Fluorine atoms that are in the equatorial positions have bond angles of 102 degrees.
Meanwhile, the axial ones are 173 degrees, which are slightly different from the trigonal bipyramidal molecular geometry that leads to a see-saw shape.
The lone pair of electrons in the central atom that leads to the changing of the bond angle. It’s from the bond angles of 120 to 102 degrees for the equatorial fluorine atoms.
What’s more, 173 degrees instead of 180 degrees for the axial fluorine atoms.
Polarity
Once you know the Lewis Structure and molecular geometry of specific compounds, it is easier to see the polarity of the molecule.
One lone pair in the central sulfur atom compound and the four bonding pairs of electrons can lead to the asymmetric distribution of electrons on the central atom.
The shape of the molecule appears like a see-saw. Two fluorine atoms are able to cancel each other in their dipole moment. However, the rest of the fluorine atoms cannot do it in their dipole moment because of the arrangement of the electron.
SF4 Applications
SF4 is used in making the water as well as oil repellent material. The fluorinating agent is also for making pesticides and lubricity enhancers.
SF4 is highly toxic through inhalation, and it irritates the skin, eyes, and mucous membranes. (1) The SF4 reacts intensely with water and acids as it yields toxic fluoride and sulfur oxide fumes. It is an acidic solution.
How To Identify the Number of Electron Lone Pairs on the S Atom in S4
SF4 has the central atom S attached to the four atoms through the four single bonds. It uses the four valence electrons and the remaining two valence electrons that remain one lone electron pair. In other words, it means that the SF4 S atom has one lone electron pair.
Keep in mind you need to know the number of valence electrons first, Lewis structure, molecular geometry, bond formation, and other properties. There are eight of the thirty-four valence electrons utilized in a covalent bonding pair. It leaves the rest of the valence electrons behind.
Through this, you will understand the properties and the model better for sulfur tetrafluoride.
References:
- https://pubchem.ncbi.nlm.nih.gov/compound/Sulfur-tetrafluoride