Nitrogen Dioxide (NO2) is one of the covalent compounds containing a central nitrogen atom bonded to an oxygen atom and double-bonded to another oxygen atom.
Our team spent a few hours researching to give you information about the NO2 Lewis structure.
Table of Contents
Understanding The NO2 Lewis Structure
One nitrogen atom and two oxygen atoms make up nitrogen dioxide. [1] Nitrogen belongs to group 15 elements in the periodic table with five valence electrons, while the oxygen atom has six electrons.
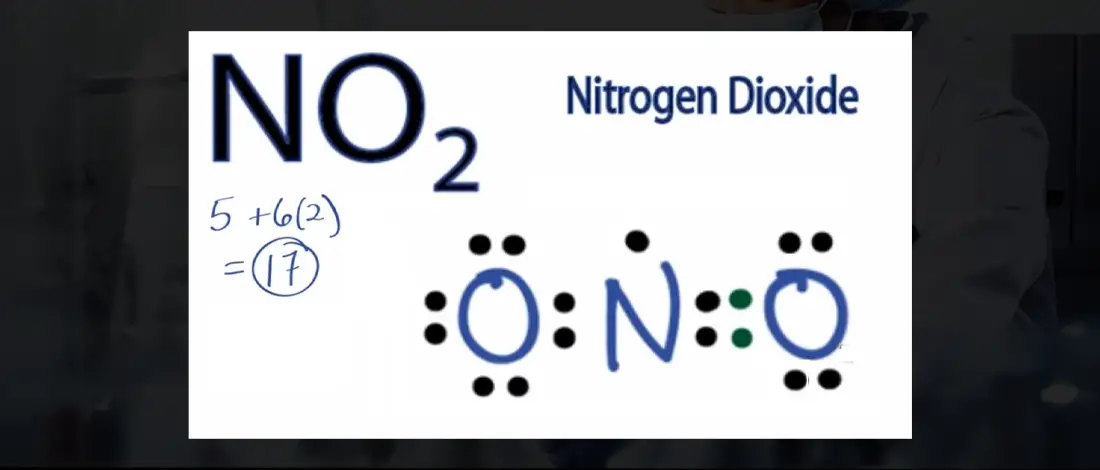
The nitrogen atom is in the center, surrounded by Oxygen atoms. We’ll now use dot structures to encircle the electrons with valence electrons. There are 17 valence electrons in the Lewis structure of NO2 – with an unpaired electron that is not stable because of the odd number of electrons.
Because we have a triatomic atom, our structure will contain a center atom connected to terminal atoms. After the central atom selection, you can now assign lone pairs to the atoms. Atoms need to achieve octet as covalent bonds are formed when atoms share their valence electrons with other atoms.
How To Draw The Lewis Structure of NO2
- Calculate the total number of valence electrons. one nitrogen atom. Two oxygen atoms make up nitrogen dioxide. The valence shell of Nitrogen has five electrons, while in the valence shell of an oxygen atom, there are six electrons. Resulting in a total number of 17 valence electrons.
- Draw the compound’s atomic structure. The least electronegative atom in greater compounds is the center atom.
- Assign lone pairs to the atoms. The illustration already has two N-O bonds. So six valence pairs remain.
- Label the six valence pairs as lone pairs. Oxygen will take three lone pairs. According to the Octet Rule, atoms need to form bonds until they reach eight valence electrons.
- Assemble electron pairs such that each atom has at least one single bond. Form two single bonds, one for each atom.
- Place the remaining electrons. Move electron pairs around to establish triple or double bonds until the atom completes the octet rule. A double bond is formed when an unbonded pair from one oxygen atom is transferred to Nitrogen.
Its Properties & Structure

Hybridization
NO2 hybridizes with sp2. Nitrogen dioxide has two sigma bonds and a single electron.
Because NO2 has 17 valence electrons, it is an odd-electron system. The central atom N has less charge than the central atom O.
As a result, N has a ‘+’ charge. As a result, the oxidation state is positive, and the lone electron will participate in the hybridization process. As a result, NO2 hybridization is sp2.
Molecular Geometry
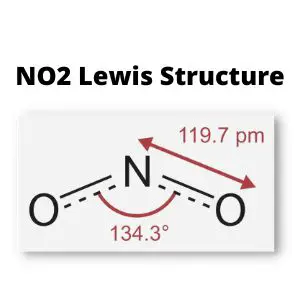
According to the VSEPR theory, The molecular structure of NO2 (Nitrogen Dioxide) is bent. However, there are a few exclusions in this case. There are two bonded pairs and an unpaired electron in the Lewis structure for NO2.
When we examine the nitrate ion NO2-, we notice that it contains two bond pairs, one lone pair of electrons. As a result, NO2 has a bond angle of around 134 degrees. Around 1.20 Å is the bond length.
Related Posts:
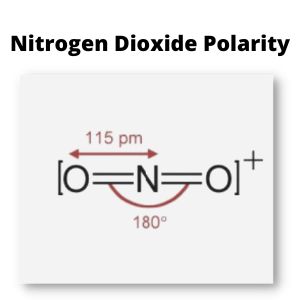
Polarity
A nitrogen atom has an electronegativity of 3.04, while the oxygen atom is 3.44.
This value indicates a difference in the electronegativity of the two atomic elements.
Although the distinction is fairly small, the asymmetrical bent molecular structure generates the net dipole moment, making nitrogen dioxide polar.
Bond Angle
The electronic form of NO2 is rather unusual. It’s more appropriately defined as a resonance hybrid of two structures, one of which includes a nitrogen atom with an odd electron. And due to the presence of an unpaired electron, Nitrogen dioxide is a free radical. The angles have been established experimentally to be 134 degrees.
MO Diagram for NO2
The Molecular Orbital Theory is a quantum mechanics hypothesis describing chemical bonding inside molecules. When you examine the electron configuration of nitrogen and oxygen atoms on the VSEPR chart, the two electrons of 1s2 in the Nitrogen atom are involved. Each of the oxygen atoms contributes two lone pairs.
The leftover electrons form the 2px, 2py, 2pz, and *2s in the p orbitals of Nitrogen and oxygen atoms.
FAQS
The molecular geometry of NO2 is trigonal planar. A double bond links nitrogen and oxygen. For electron pair geometry, the molecule has three electron pairs. Because it has an additional electron after interacting with oxygen atoms, Nitrogen has a negative charge. But, what’s the difference between molecular geometry and electron pair geometry?
There are two lewis structures in NO2. The multiple Lewis structures are referred to as resonance structures when many Lewis structures can represent the same molecule. [2]
There are 17 valence electrons in the Lewis structure of NO2. It’s unusual for a Lewis structure to contain an odd quantity of valence electrons. Compared to the nitrite ion (nitrous acid), NO2 has unpaired electrons in its Lewis structure than the nitrite ion, while NO2 has one lone pair in the nitrogen atom.
Key Takeaways
NO2 contains three pairs of electrons surrounding the nitrogen atom for molecular structure. The one-electron allows the two connecting oxygen atoms to stretch out to a 134 angle.
We use Lewis structure (Lewis dot structure) to understand the chemical bonds, the number of lone pairs, whether double or triple bonds, and how many electrons are in NO2 molecules.
Thank you for reading this far! I hope that the information provided in this article will be helpful to you.
References:
- https://www.epa.gov/no2-pollution/basic-information-about-no2
- https://courses.lumenlearning.com/boundless-chemistry/chapter/formal-charge-and-resonance/