Sulfur is used for making gunpowder, matches, fireworks, insecticides, and fumigants. Elemental sulfur is also for the treatment of some skin diseases. The principal use of sulfur can be found in the preparation of compounds.
When it comes to organic chemistry, specifically the Lewis Structure, how many lone pairs does sulfur have? Find the answer below.
Table of Contents
Determining the Number of Lone Pairs in Sulfur
The sharing of electrons allows atoms to stick together. It is the basis of covalent bonding. Some are intermediate distant, which is longer than 0.1 nm.
If you prefer 100 pm, it is where the attractive forces surpass the repulsive forces. A formation of a bond if the atoms can achieve complete s2np6 configuration.
It is this specific behavior that Lewis captured in the octet rule. You need the valence electron configuration of a constituent atom of the covalent compound.
These are essential factors to determine the structure, stoichiometry, and properties.

Identifying Lone Pairs in a Molecule
The bond pairs are a pair of electrons. One is from the central atom while one is with the atom, which is known as bonding. It participates in the bonding of the atom.
Meanwhile, lone pairs are the pairs of the electron in an atom that do not take part in the bonding of the two atoms.
To identify the lone pairs in the molecule, you have to figure out the number of valence electrons in an atom. Next, you have to deduct the number of electrons that took part in the bonding.
You have to keep in mind that the lone pairs are pairs. Therefore, if you can find one free electron that isn’t participating, it means the compound is a charge.
Related Posts:
Using the Lewis Structure
You need to determine the valence electrons per atom. You add them together with the total number of electrons basing on the overall species charge. It can either gain or lose numbers.
After which, you determine its central atom, which is the least electronegative element. You draw the single bonds that are between the central and terminal atoms.
Subtract the electrons in forming the bonds from the first step while keeping in mind that a single bond is composed of two electrons.
Around the terminal atoms, you distribute the line pairs, so they are following an octet. Subtract the number of electrons from the number you have obtained.
Put the remaining electrons in its central atom. Then, you see if it’s possible to reduce formal charges. The structure that gives you the smallest charges in magnitude is what you call the Lewis Structure.
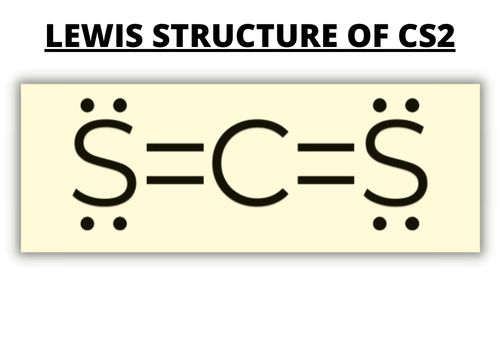
Using the VSEPR Theory to Determine Sulfur’s Lone Pairs
Using the VSEPR model, you can predict the structure of most molecules or polyatomic ions, which means the central atom is a nonmetal.
It includes structures of plenty of molecules and polyatomic ions that have central metal atoms.
The electron pairs are in bonds. Meanwhile, the lone pairs repel from each other. In other words, it adopts the geometry that puts the electron pairs far from each other as much as possible.
It is a simplistic theory, and it can’t account for the subtleties of the orbital interactions that influence the molecular shapes (1).
On the other hand, the counting procedure can accurately predict the 3D structures of a massive number of compounds.
So, How Do You Identify the Number of Lone Pairs in Sulfur?
The sulfur atom shares a bonding pair and three lone pairs. In total, it has six valence electrons.
To keep it simple: you can identify the number of lone pairs in sulfur by following these steps.
- Arrange the atoms to show specific connections.
- Determining the total number of valence electrons
- Put a bonding pair of electrons between each pair of the adjacent atoms
- Add enough electrons to each atom
- If any electrons are left over, place them on the central atom.
Thank you for reading this far! I hope that the information provided in this article will be helpful to you.
References:
- https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_Chemistry_-_The_Central_Science_(Brown_et_al.)/09._Molecular_Geometry_and_Bonding_Theories/9.2%3A_The_VSEPR_Model


