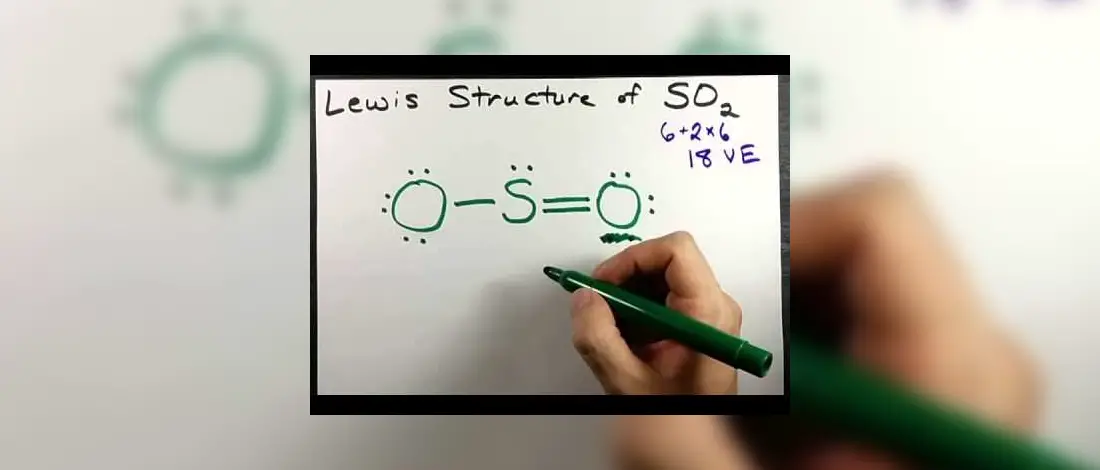Understanding the Lewis Structure of a compound element is necessary to understand how the molecule and the atoms came up with its shape, geometry and how it emerged into such. You will better understand the origins of SO2 from Oxygen atoms and Sulfur and its uses if you can know its source.
Table of Contents
Sulfur Dioxide (SO2): A Brief Description
Sulfur dioxide, or Sulphur Dioxide as spelled in Commonwealth English, is a toxic chemical compound that causes the smell of burning matches.
Normally, this originates from volcanic activities or produces as by-products to extract copper and incinerating fossil fuels that are sulfur-bearing.
This is a colorless inorganic gas but has a weak acid solution when mixed with water.
Sulfuric acid is a toxic gas that can cause respiratory health problems.
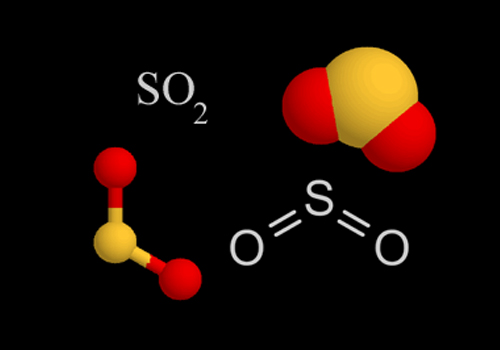
What You Need To Know

Lewis Structure
Drawing Lewis Structure of SO2 or Sulfur Dioxide will first require you to get the total valence electrons on both Sulfur and Oxygen. Once that is done, all should be arranged on the outermost shell of Sulphur. There are six valence electrons for the two, Sulfur and Oxygen, and based on the Octet Rule, if there are lone pairs or nonbonding electrons, it can lose or gain and share atoms until it is finally complete.
Hybridization
With Sulfur Dioxide, Oxygen atoms contain one sigma bond and two lone pairs the same as Sulfur atoms. Therefore the hybridization of SO2 is SP2. You will clearly see the transformation and explanation after you draw the skeleton structure of the molecule where the sulfur atom and oxygen atoms create a double bond.
Molecular Geometry
To understand Molecular Geometry better, understand the positions and number of electrons distributed among Sulfur and Oxygen.
A sulfur atom on the outer level has six electrons, and four are used by Oxygen for each bond.
That leaves a total number of ten electrons in five pairs.
One lone pair is left while two double bonds form as a unit, making the Bent or V shape.
Molar Mass
You can calculate the molar mass by using the usual formula n=N/NA (then N=n*NA). N is the number of atoms, while A stands for Avogadro’s constant, which is 6,023×10^23. This will give us the result of 64.066 g/mol as the molar mass of SO2.
Electron Geometry
The electron geometry of SO2 is trigonal planar. This is not similar to molecular geometry, where only the total number of atoms is considered to determine its shape. Electron geometry includes all the pairs of electrons, even the lone pairs. With SO2, the pairs of bonding electrons are arranged at an angle of 120 degrees.
Polarity
There is an imbalance charge across other atoms in the molecule, making Sulfur Dioxide polar. There is polarization because Sulfur pulls the charge of the molecule to its side while gaining partial negative charge and not Oxygen since it is the least electronegative atom, making SO2 a polar molecule.
Bond Angle
Looking at the molecular geometry of SO2, the bond angle is at 120°. This means that the o-orbitals are also almost perfect SP2 orbitals. Although this may be confusing since there are different bonds, a double bond and a single bond is present, including triple bonds of the molecule, it still comes up with the same bond angles on all.
Related Posts:
5 Step By Step Construction of SO2's Lewis Structure

1. Find The Total Valence Shells’ Electrons
The two molecules found on this compound belong to the VIA elements; therefore, they have six valence electrons, multiply it with the number of atoms found on each molecule and then add the products.
- Valence electrons from two oxygen atoms = 6 * 2 = 12
- Valence electrons from one sulfur atom = 6 * 1 = 6
- Total valence electrons = 12 + 6 = 18
2. Determine The Total Electrons Pairs
Once you find the number of electron pairs, you divide the total valence electrons by two. You have six
SO2 total electron pairs /2= 12/2= 9
3. Center Atom Selection
In finding which should be the center atom, the one with the highest valence will be considered. For SO2, Sulfur has the higher valence among the two atoms. You will see the bond order, single bonds form, and double.
4. Mark Lone Pairs On Atoms
In the S-O bond, you will see that there are two of them.
In marking a lone pair, determine how many are left.
Begin with the valence electrons on the outside of the oxygen atom, which should show two bonds.
There will be six valence electron pairs from Oxygen atoms which will leave one valence electron pair left.
That will be marked as a lone pair under the Sulfur atom.
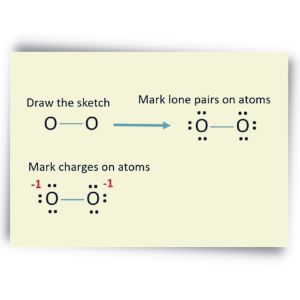
5. Check The Stability And Minimize Charges On Atoms
In order to find out what the most stable structure is, the charges of the atoms should be established in their lowest form possible. Since the lone pair is already marked and those bonded, it will be easier to mark the charges of each atom. It will use this formula:
Formal Charge = No. of valence electrons – No. of Bonds – 2 X (No. of lone pairs) = 6-2-(2×2) = 0
Other Similar Lewis Structures of So2

There are many similar Lewis Structures of SO2 depending on the shape, geometry, and lone pairs. The SO2 Lewis Structure of water, hydrogen sulfide, nitrogen dioxide, and ozone has the same shape, which is bent. Hydrogen sulfide, water, sulfur dioxide, and nitrogen dioxide have two sigma bonds. Lastly, Ozone and SO2 have one lone pair on their central atom.
Sulfur Dioxide’s Effect on Humans
This is a useful chemical, but it has harmful and toxic effects on a human being. Sulfur Dioxide has a negative reaction when inhaled. The respiratory tract has high infection possibilities, resulting in coughing and secretion of mucus, leading to serious cases of chronic bronchitis or asthma.
FAQS
The best Lewis Structure would be where the two oxygen atoms have double bonds to make their formal charges zero and the formal charge of Sulfur.
The electron geometry of Sulfur Dioxide is a trigonal planar shape where the bond angle of the three pairs of electrons is lined at 120 degrees.
Final Thoughts
Like other compounds, understanding and drawing the Lewis Structure will help you fully grasp how the molecular geometry, properties, and other components came to be. With SO2 Lewis Structure, the central atom is the central sulfur atom because of the higher valence of the sulfur atom than the oxygen atom.
The SO2 Lewis Structure provides the best explanation of how the sulfuric acid(1) transformed into such after dissecting the bonds of Sulfur and Oxygen. This can be a hazard to one’s health, but this is also a useful compound if you know how to use it properly.
Thank you for reading this far! I hope that the information provided in this article will be helpful to you.
References:
- https://pubchem.ncbi.nlm.nih.gov/compound/Sulfuric-acid