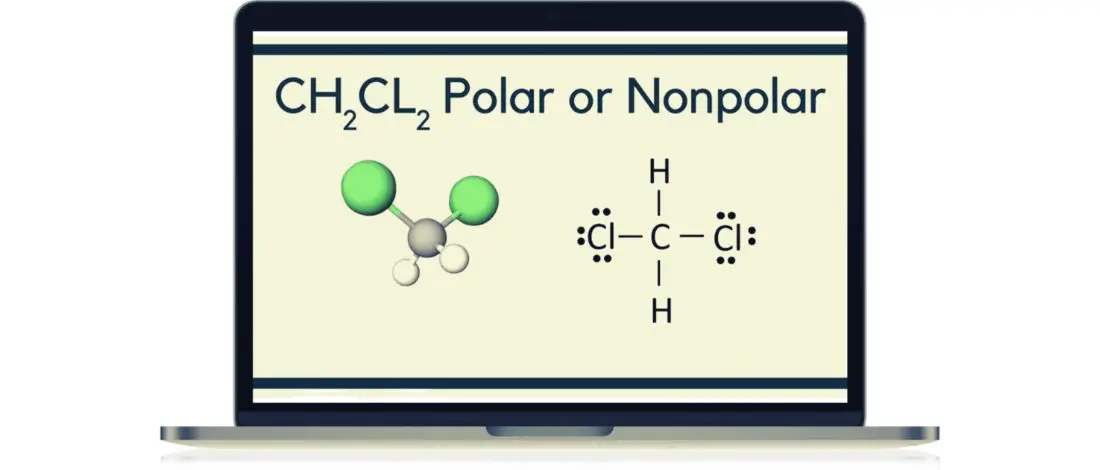
Whether CH2Cl2 is polar or nonpolar is still a question of many.
Do we see any separation of any electronic charge in this compound, which led the molecule to have both positive and negative ends?
We will explain how the molecule from the periodic table changes because of molecular geometry and electronegativity.
Table of Contents
Polarity or Nonpolarity of Dichloromethane
First, let us describe the chemical formula CH2Cl2, also known as Dichloromethane.
It is a clear, colorless, volatile liquid with a slightly sweet odor that commonly originates from macroalgae, volcanoes, oceanic sources, and wetlands.
This would be associated with electricity, magnetism, and electronegativity when talking about polarity in a usual scenario.
This is not one of the usual scenarios, though. Still, Dichloromethane, also known as Methyl Chloride, develops a net dipole moment across C-Cl and C-H bonds.
The chemical bond results in a net 1.67 D dipole moment, thus making it a polar compound.
Read: CH3OH – Polar or Not?
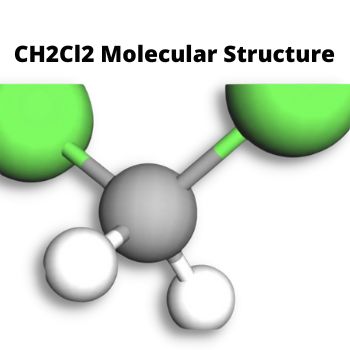
CH2Cl2 Molecular Structure
We look at the Lewis Structure of Dichloromethane or CH2Cl2 to determine if chemical reactions change its structure.
Lewis structure will help us understand, based on the octet rule, the structure of a specific compound with eight electrons in its outer shell to be stable or inert.
In the compound Dichloromethane, you will find Carbon atoms with 4 electrons, and Hydrogen atoms have 2 electrons in their neutral form.
The two atoms need additional electrons to complete the bond formation.
Chlorine atoms, on the other hand, have 17(seventeen) electrons distributed around their nucleus.
However, only 7 of those are valence electrons that are on the outer shell.
Therefore, there are a total of 20 valence electrons. Out of 20 valence electrons, 8 electrons took part in the bond formation.
How to Determine CH2Cl2 Polarity

Shape
You can also determine the polarity of a compound with its shape. If the dipole moment on each molecule does not cancel out, then that is a polar compound.
However, a nonpolar molecule can have polar bonds within it, but the dipoles of these valence electrons get canceled by each other due to the symmetric shape of the molecules.
Electronegativity
The one with the partial negative charge in a polar covalent bond is the more electronegative atom. The electron distribution is more polarized, and the partial charges of these atoms become more prominent. Some electrons have lower electronegativity(1), such as the Hydrogen atom, compared to the electrons found on the chlorine atom.
Related Posts:
- Determining the Number of CS2 Double Bonds
- Determining the Lone Pairs of Sulfur
- Determining the Lone Pairs of Electrons on the S Atom in SF4
- Determining the Polarity & Nonpolarity of a Molecule
- Electron Pair Geometry vs Molecular Geometry
Dipole Moment
What made us say CH2Cl2 is a polar compound?
In the case of Dichloromethane, the distribution of positive and negative charges of the molecules of all the bonding atoms are as follows, Hydrogen=2.2, carbon atom =2.5, and chlorine=3.1.
Electronegativity has an unequal distribution, partly because of Hydrogen molecules, which are lower than the two bonds.
There is symmetry in the compound despite Hydrogen being less electronegative than a carbon atom, causing it not to cancel out; hence there are dipole moments in the compound.
FAQS
Methylene Chloride has many uses after the bonding process of the highly volatile molecules.
It is part of the food industry and beverage manufacturing industries as an extraction solvent. It is used in processing spices, hops extracts for beer, and other flavorings.
It extracts chemicals from plants or foods for steroids, antibiotics, and vitamins. These are also efficient in cleaning medical equipment without causing corrosion issues.
CH2Cl2 is a covalent bond, where central carbon is hybridized, and all the four bonds in the compound are called SP3.
We can also take note that the molecular geometry of CH2Cl2 is tetrahedral with the shape trigonal pyramidal. The physical properties of CH2Cl2, the compound from the molecule bonds formed, are the following.
It has a density of 1.3226g/cm3 with a molecular weight of 84.96g/mol. Its boiling point is at 39.60C, and its melting point is -97.60C.
So, Is CH2Cl2 Polar or Non-polar?
In chemistry, the answer to the question is a definite yes because of many reasons.
Firstly, it has polar molecules shaped trigonal pyramidal.
The second reason is that the geometry of the compound is tetrahedral, which according to chemistry, has a central carbon atom surrounded by four other atoms.
The third is the electronegativity between C-H and C-Cl is 0.4 and 0.6, respectively.
Though a Chlorine atom is nonpolar, the polar molecule emerges after valence electrons of nonpolar molecules bond its properties.
So, it is indeed a fact that even if there are nonpolar molecules, but the bonds do not cancel, and the geometry is showing polarity, then CH2CL2 is polar.
References:
- https://www.thoughtco.com/definition-of-electronegativity-604347