Three iodine atoms in a polyhalogen ion, anion, formed when the Aquarius solutions iodine and iodide salt are combined.
To find out what the structure of a Triodide ion is, we need to get the full concept of its physical properties, shape, and hybridization. Let’s get the valence electrons and the central atom to start it off when dissecting the I3 Lewis Structure.
Triiodide Ion (I3): A Brief Description
There are three iodine molecules, hence the name Triiodide (1) or better known as I3- which is also a polyatomic ion and a negatively charged ion of -1. The given molecule has a non-reactive property towards starch which makes it useful in identification due to the identifiable blue-black color it produces.
It also has a molecular weight of 380.7134. There are seven valence electrons found in this ion which has an exergonic balance that leads to the formation of the ions. There is a positive flow of energy on the charged molecule from the system to the environment.
What You Need To Know
Lewis Structure
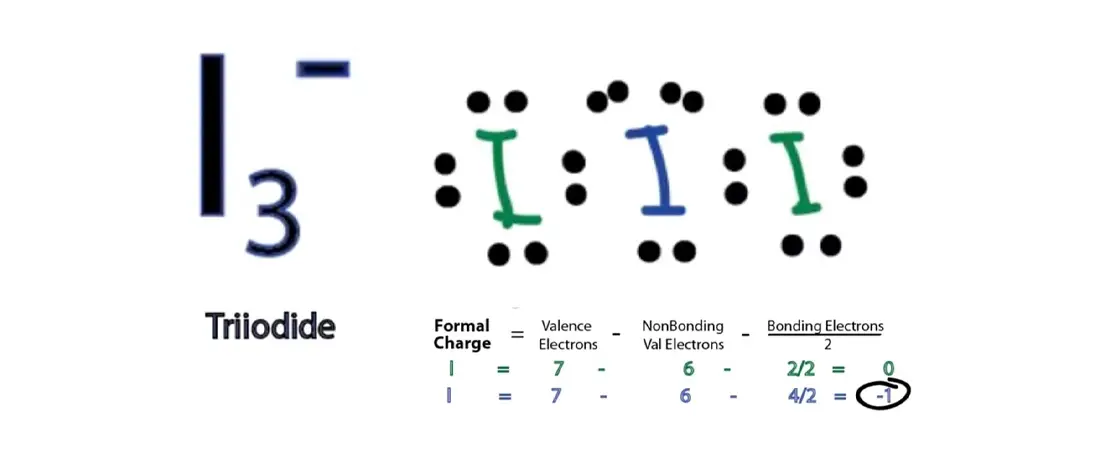
The Lewis Structure of I3- will give you a better understanding of how the chemical bonding is completed. One of the three iodine molecules has an extra negative charge among all the atoms. Because of that, considered an extra electron, we have 3 lone pairs and 2 bond pairs that result in its steric number 5 and is number 53 on the periodic table. Also, the formal charges of the molecules should be at their lowest value.
Hybridization
To find out what the hybridization of I3- is, we need to use the simple formula in finding hybridization number, which is;
Number of valence electron + monovalent + (negative charge) – (positive charge)/2
In an iodine molecule, you will find seven valence electrons. There are two monovalent atoms, and the value of the net negative charge will be 1 since it has a negative charge.
The value should be:
7+1+2/2
=10/2
=5
Since the hybridization number is 5, it makes the SP hybridization sp3d.
Molecular Geometry
Looking at the Lewis Structure of I3-, the central iodine shows 3 lone pairs with equatorial positions because of the repulsion of the valence electrons. Now, the angle of the bond became 1800 when the two iodine atoms were axially placed. The central iodide atom has a negative charge. Therefore, it makes the shape of the I3- ion symmetrical and linear.
Molar Mass
You can calculate the mass of the molecules depending on the weight of the element. In this case, the atomic weight of iodine is 126.90447, and there are 3 atoms found in this molecule. Therefore, the molar mass of i3- is 380.713410.
Electron Geometry
One atom of the three is negatively charged, and this also gives two bond pairs and 3 lone pairs of valence electrons, making the molecular geometry linear.
Related Posts:
- BCl3 – Polar or Nonpolar?
- SF4 – Polar or Nonpolar?
- BRf3 Molecular Geometry
- CO2 Lewis Structure
- BF3 Lewis Structure
Polarity
All the values of the I3 ion are negative as it only has one electron. If a molecule has a partially positively charged end and a partially negatively charged end, then it will have polar bonds, but this is not the case for I3-, therefore, it is not polar or nonpolar.
Bond Angle
The bond angle of I3- was formed when the two other iodine molecules bonded with I- ion. The value of this is 180 degrees celsius. Since the three molecules have seven electrons that are non-bonding, the other two iodine atoms formed the bond instead.
Shape
The shape of I3- ion is linear and also symmetrical. The iodine molecules bonded axially in a linear fashion. There are two atoms sharing valence electrons out of three lone pairs in this molecule, and based on the VSEPR theory, I3- has three equatorial lone pairs which have also bonded to the central iodine atom.
How to Draw the Lewis Structure for I3
When drawing Lewis Structures, we first count the valence electrons with the use of the periodic table. In the periodic table, iodine is the seventh group with the atomic number 53, and it has seven electron valence.
In this Lewis Structure, you will see three molecules with an extra electron that has a negative charge; therefore, the computation will be: 7×3+1=22
We must follow the Octet rule, so it will be the Central Atom sharing electrons to neighbouring Iodine atoms from its eight electrons in its outermost shell, leaving six electrons that will form the non-bonding lone pairs.
The last thing to make sure is that all molecules maintain the lowest possible formal charge.
Read: C2H2 Lewis Structure
FAQS
I3- is not bent because it is in a different electronic environment. It is bound to two oxygen valence electrons that cause repulsion on the outer shell since it pulls electrons on the outer orbit.
No. I3- Lewis Structures shows that this only has one negatively charged electron, and even the best Lewis structure will not show any dipole moment. I3- is not a polar molecule or a nonpolar molecule.
Key Takeaways
The I3 Lewis Structure, just like any Lewis Structures, will provide enlightenment on how to understand the structure of a molecule better. The seven valence electrons of iodine created the Triiodide ion, though this is a confusing process of chemical bonding, especially for beginners.
We now know how to deal with transforming non-bonding molecules and always ensure that a molecule’s formal charge is kept at the lowest form. All these will be taken into consideration since we have a better understanding of how the structure works.
Thank you for reading this far! I hope that the information provided in this article will be helpful to you.
References:
- https://pubchem.ncbi.nlm.nih.gov/compound/Triiodide-Ion