Taking an organic chemistry class needs a lot of preparation, and reading textbooks isn’t enough. A molecular model kit is an essential tool for any student as it helps you understand chemistry concepts better.
The question is, what’s the best organic chemistry model kit to buy? After hours of testing, connecting, and building each kit, here’s our ultimate list.
OUR #1 RATED
Dalton Labs
- 123 to 496 parts available
- Builds basic organic compounds
- Great price value
- Includes p and pi orbital
- Software and user guide
OUR #2 RATED
Old Nobby
- Bonus learning guide
- Handy link remover
- Swiftly connects between atoms
- High-quality items
- Beginners to graduate students
OUR #3 RATED
Duluth Labs
- Affordable price
- Durable
- High-grade
- Includes molecular tool
- Solid container
Top 10 Organic Chemistry Model Kits in 2023
1. Dalton Labs
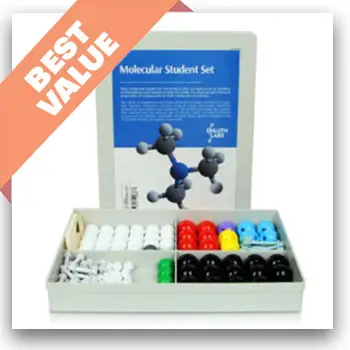
You’re getting a conventional ball-and-stick molecular model kit that provides you with color-coded atoms to fit the universal standards.
Most of the items in the model kit are carbons, hydrogens, and bonds. The single bonds are short and rigid. Meanwhile, Dalton labs provide you with longer and flexible items for double and triple bonds.
What makes Dalton Labs model kit better than similar model kits is that it gives aspiring scientists small, paddle-shaped items for the p and pi orbitals.
Although it’s not required, these assist the students in understanding electrons better in a provided molecule.
The model kit delivers good value, considering it offers 306 parts in total. It also gives the students access to 3D molecular modelling software.
Depending on your child’s needs, the model kit comes in various sizes, starting from 123 to 496 parts (fullerene models).
PROS
- 123 to 496 parts available
- Builds basic organic compounds
- Great price value
- Includes p and pi orbital
- Software and user guide
CONS
- Only offers eight oxygens
- Few mid-size white single bonds
2. Old Nobby
Old Nobby Molecular Model Kit delivers organic, inorganic, and functional groups for easy chemistry learning. It fits both beginners and advanced students for versatility.
For first-time users, the modeling kit provides you with an eight-page instructional guide so that you can identify the atoms and connectors.
The Old Nobby molecular kit is a 239-piece kit, which falls short compared to the 306-piece Dalton Labs model kit.
The kit provides you with color codes to visually help you understand the structure and compounds better.
The high-quality atoms and bonds allow you to swiftly connect and disconnect, so you can build complex structures within minutes to avoid finger fatigue.
The manufacturer threw in a bonus link remover to make dissembling easy and fast.
PROS
- Bonus learning guide
- Handy link remover
- Swiftly connects between atoms
- High-quality items
- Beginners to graduate students
CONS
- Low-quality box
- Requires larger container
3. Duluth Labs
Duluth Labs is another conventional ball and stick kit, similar to other model kits on the list. It comes with 125 items, and it does not have a lone pair or p orbital items.
It’s a good option for a tight budget as it’s one of the cheaper ones on the market. However, it can only build only several models at once, which may not fit for classes.
It comes in a rigid plastic box with a lid to secure it. Unlike the Old Nobby chemical structure model kit, the container can hold them without them falling over, which is a neat feature.
In total, it gives you 54 atoms for your molecular structures and functional groups. It comes with a molecular tool as well, allowing youtube to disassemble molecules and the atom connectors.
PROS
- Affordable price
- Durable
- High-grade
- Includes molecular tool
- Solid container
CONS
- May lack items for classes
- No p orbital items
4. Molymod
Molymod molecular kit is cheaper than Duluth Labs, making it the cheapest product on the list. If you’re looking for a budget-friendly option, taking advantage of this 113-piece kit makes a good deal.
While it isn’t as extensive as other brands, it does cover the essentials for basic modeling in your organic chem classes.
On the other hand, because it lacks items, you cannot build multiple molecules simultaneously for teachers. It is especially true for larger molecules, such as sugars.
The plastic container comes with four compartments, so it’s easy to organize for storage.
PROS
- For grade seven to twelve
- Durable plastic
- Well-organized kit
- Easy to put together
- Quick to pull apart
CONS
- Connections become loose
- Lacks items
5. Happy Atoms
The Happy Atoms molecular model kit allows you to build, scan, and identify the molecules and elements. It’s a 17-atom chemical structure set, including the following atoms: 8 H, 3 C, 2 N, 2 O, 1 NA, and 1 cl.
What makes Happy Atoms molecular model kit the go-to option for some buyers is its free downloadable application, which allows you to take a snap with your smartphone to scan the molecule you built.
It analyzes the atom’s size, shape, and markings.
The application shows its composition, usage, properties, and so much more. It recognizes over 10,000 molecules in its modelling kit.
PROS
- Free happy atoms app
- High-grade items
- Delivers 17-atom pieces
- Durable
- Includes instruction manual
CONS
- App malfunctions
- May be too expensive
6. Molecular Visions
Molecular Visions molecular model kit is different from the other model kits on the list as it does not have the typical ball-and-stick structure. Instead, it uses a push structure system, which is similar to the Kinex set.
The molecule model kit by Molecular Visions is quite costly, and you’re getting it in a small plastic box.
However, what makes the molecular model kit neat is the included guidebook. Students and teachers look at it as a mini organic chem course because of the value it brings.
You may find the items harder to use than the traditional ball and stick molecular model structure, though. It’s a little challenging to put the things back into their case as you need to invest time to fit. You can always change the original box to make it more convenient.
PROS
- Value-packed guidebook
- Quality items
- Durable
- Geometrically accurate
- Hard plastic kit
CONS
- Difficult to put together
- Some items are too small
7. LearnOn
Despite being one of the more affordable molecular model kits on the list, its quality is far from being the worst. Upon purchasing LearnOn molecular model kit, you’re getting a high-value eight-page illustrated user guide for your reference.
The package comes with a total of 140 items. To be precise, you are given fifty high-quality atom parts and ninety links in this chemistry molecular model kit for building structure.
Like how the molecular model kits are, the atoms are color-coded to fit the universal standards, making identification easy. This educational molecule kit also gives you a link removal tool.
PROS
- Affordable kit set
- Durable set
- Quality modeling kit
- Sturdy plastic box
- Includes link removal tool
CONS
- The lid doesn’t stay shut
- Lacks standard items
8. Snatoms
Snatoms chemistry molecular model kit is one of the more premium model kits on the list. If you’re willing to pay the cost for the best quality, Snatoms chemistry molecular model kit is a good choice for chemistry.
Unlike the molecular model kits ones, the atom pieces are magnetic. Snatoms believe that having the atoms stick together using magnets for models provides a more realistic understanding of how it looks for teachers and students.
The molecular model kit enables you to have lesser items in the molecular model kit as you do not need the stick pieces. Due to this, the organic chemistry kit only comes with 74 pieces in the box.
This educational molecule has magnetic carbon atoms, but you’ll find small black bars as well for double and triple bonds.
PROS
- Magnetic atoms in set
- High-caliber modeling kit
- Durable
- Fewer pieces
- Fast assembly
CONS
- Expensive
- Might be too small
9. University Chemistry Co.
The University Chemistry organic chemistry kit gives you 273 pieces, including the paddle-shaped lone pair or p orbital pieces.
However, it is slightly more expensive than the Dalton Labs Kit organic chemistry model, which may put some buyers off.
Both the atoms and the bonds are easy to connect and disconnect in these molecular model kit structures.
The organic chemistry model kit gives you 115 atoms across eight different elements, which are the following: carbon, hydrogen, oxygen, nitrogen, metal, phosphorus, chlorine, and sulfur.
The box is quite durable, and it’s spacious enough to keep the pieces together after usage.
PROS
- Includes p orbital piece
- Easy to connect and disconnect
- High-class piece set
- Durable storage
- Easy to organize
CONS
- Needs more smaller-sized ones
- Long bonds need more flexibility
10. Atomic Architect
The Atomic Architect organic chemistry model is a 124-piece model set, which includes fifty-four atoms and seventy bonds. The conventional ball and sticks activity chemistry model kit is ideal for high school to graduate-level students.
The organic chemistry model kit gives you atom centers, bonds, and orbitals. The pieces are easy to connect and disconnect as well. For convenience, you’re getting a disassembly tool you can use to remove bonds.
The pieces are color-coded as well to fit the universal standards. The organic chemistry model kit storage box is designed for grade-A construction and easy fit.
PROS
- Nice small-scale visual aid
- Affordable price range
- Grade-A parts in set
- Durable set container
- Easy to take apart
CONS
- Not for multiple bonds
- Not for larger molecules
Why Should I Use This Kit?
Molecular model kits for chemistry make it easier to grasp organic chemistry, especially for students with no prior experience on the matter.
Organic chemistry is different from general chemistry as it does not entail all math and equations. It’s similar to learning a new language, and learning the grammar of organic chemistry is highly dependent on how you understand molecular geometry, electron orbitals, and spatial orientation.
It enables you to visualize molecules in 3D, so it’s easier for students to understand the molecular structures better. (1)
Buying Guide
Pricing
An organic chemistry model kit isn’t expensive. Certain models offer a money-back guarantee if you’re looking for the best bang for your buck.
You can find the best molecular model kits moderately priced. The fewer the pieces, the cheaper the models are. For premium kits with magnetic atoms, they are fewer but costs more. There are several chemistry sets for adults and science kits for teens that are affordable yet efficient.
Portability
The best molecular model kits have sturdy plastic containers that make it easy for students and the professor to carry it around. Avoid purchasing molecular model kits for chemistry with boxes that do not close securely, as there’s a chance the pieces might fall off.
If you’re looking for models for portability, choose chemistry kits with smaller atom pieces. You may also add a USB microscope to your collection.
Related Posts:
Usability
If you’re a grade 7 student, you might not need to purchase chemistry models to build complex structures.
For a teacher, you may need to invest in larger chemistry kits that can satisfy classes.
Make sure the atom parts are enough to build molecular structures for your students.
Also, pieces that are easy to connect and disconnect, so you may avoid buying the push-clip system chemistry kits.

Number of Pieces
Purchase a chemistry set and kit that caters to your needs. While a 120-piece might be cheaper, it may not have enough atom pieces.
If you’re a student in earlier grades, fewer pieces are enough for your organic chemistry subject. However, graduate-level should invest in a 300-piece or a 400-piece chemistry educational molecule model kit. We suggest you add a good pair of lab goggles to your purchase.
Special Features
A molecular model kit for chemistry provides you with unique features, such as a convenient application to make it easier for your student to understand the molecular structures better. However, these are pricier than the conventional ones. You may opt for magnetic pieces as well for convenience. We also recommend picking a periodic table poster and a reliable Chemistry textbook for a deeper understanding.
And Our #1 Choice for Organic Chemistry Model Kit is…
At the end of our research, the Dalton Labs molecular model kit comes up on top. It provides the best value among the other organic chemistry kits and fits the requirement of our buying guide.
It might be on the cheaper end, but it gives you a 200-piece kit with an option to upgrade to a kit with a higher number of pieces. Dalton Labs also delivers pieces that represent orbitals to develop your learning further.
The pieces are top-tier, and it comes with a sturdy case.
Thank you for reading this far! I hope that the information provided in this article will be helpful to you.
OUR # 1 Recommendation
Dalton Labs Molecular Model Kit
- 3D Modeling software
- Colored Printed Guide
- Periodic Table in set
- Sturdy Plastic Case